Molecular mechanisms of action of herbal antifungal alkaloid berberine, in Candida albicans - PubMed (original) (raw)
Molecular mechanisms of action of herbal antifungal alkaloid berberine, in Candida albicans
Sanjiveeni Dhamgaye et al. PLoS One. 2014.
Abstract
Candida albicans causes superficial to systemic infections in immuno-compromised individuals. The concomitant use of fungistatic drugs and the lack of cidal drugs frequently result in strains that could withstand commonly used antifungals, and display multidrug resistance (MDR). In search of novel fungicidals, in this study, we have explored a plant alkaloid berberine (BER) for its antifungal potential. For this, we screened an in-house transcription factor (TF) mutant library of C. albicans strains towards their susceptibility to BER. Our screen of TF mutant strains identified a heat shock factor (HSF1), which has a central role in thermal adaptation, to be most responsive to BER treatment. Interestingly, HSF1 mutant was not only highly susceptible to BER but also displayed collateral susceptibility towards drugs targeting cell wall (CW) and ergosterol biosynthesis. Notably, BER treatment alone could affect the CW integrity as was evident from the growth retardation of MAP kinase and calcineurin pathway null mutant strains and transmission electron microscopy. However, unlike BER, HSF1 effect on CW appeared to be independent of MAP kinase and Calcineurin pathway genes. Additionally, unlike hsf1 null strain, BER treatment of Candida cells resulted in dysfunctional mitochondria, which was evident from its slow growth in non-fermentative carbon source and poor labeling with mitochondrial membrane potential sensitive probe. This phenotype was reinforced with an enhanced ROS levels coinciding with the up-regulated oxidative stress genes in BER-treated cells. Together, our study not only describes the molecular mechanism of BER fungicidal activity but also unravels a new role of evolutionary conserved HSF1, in MDR of Candida.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
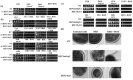
Figure 1. Antifungal potential of BER (a) Growth curve of WT C. albicans cells at 100, 150 and 200 µg/ml, (b) serial dilution assays in solid (left panel) and liquid medium for testing BER susceptibility of C. albicans and non albicans species.
(c) Serial dilution assays of CDR1 (Gu5) and MDR1 (F5) overexpressing and (d) their deletions strains in presence of BER.
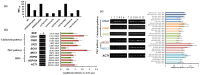
Figure 2. TF mutant library screening (a) Serial dilution assays of TF mutant strains in the presence of BER, (b) end point comparative RTPCR of HSF1 (gene deleted in JMR044) in WT strain (DAY286) in presence and absence of BER.
Figure 3. HSF1 conditional mutant is susceptible to various antifungal drugs (a) susceptibility WT, HSF1 conditional mutant and HSF1 heterozygous for BER (b) different classes of antifungal drugs; FLC, CAS, TRB, AMB, and their combination with BER, (c) CW perturbing agents; CFW, CR, SDS (d) TEM images of WT, HSF1 conditional mutant and HSF1 heterozygous in presence of BER.
Figure 4. Effect of BER on CW integrity mutants (a) serial dilution assay of calcineurin and MAP kinase pathway and HSP90 gene deleted to evaluate BER MIC50, (b) end point comparative RTPCR of genes involved in CW integrity in WT C. albicans cells in presence and absence of BER, (c) and in HSF1 conditional mutant lane indicates 1: WT, 2: HSF1 TET/hsf1, 3: HSF1/hsf1, 4,5,6,: +Doxy, 7,8,9 :+BER, 10, 11, 12: +Doxy+Ber.
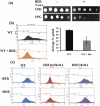
Figure 5. BER treatment results in dysfunctional mitochondria (a) growth of C. albicans cells in non-fermentable carbon source (glycerol) in presence of BER (b) MTR labeling of the active mitochondria by FACS in C. albicans WT cells in presence and absence of BER, bar graph representing number of events gated (c) MTR labeling were also done in WT, HSF1 conditional mutant and HSF1 heterozygous strains in presence and absence of BER.
Figure 6. Determination of endogenous ROS generation by BER and induction of apoptosis (a) (upper panel) bar graph representing relative fluorescent units when cells were treated with DCFDA in presence and absence of BER, AA is added to revert the ROS production, (lower panel) fluorescent microscopy images of WT C. albicans cells labeled with DCFDA, (b) Cytometric determination FITC Annexin V labeling in WT cells treated with BER.
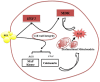
Figure 7. Model depicting pathways affected by BER treatment in C. albicans.
Similar articles
- Non-heat shock responsive roles of HSF1 in Candida albicans are essential under iron deprivation and drug defense.
Nair R, Shariq M, Dhamgaye S, Mukhopadhyay CK, Shaikh S, Prasad R. Nair R, et al. Biochim Biophys Acta Mol Cell Res. 2017 Feb;1864(2):345-354. doi: 10.1016/j.bbamcr.2016.11.021. Epub 2016 Nov 24. Biochim Biophys Acta Mol Cell Res. 2017. PMID: 27889440 - In vitro effect of malachite green on Candida albicans involves multiple pathways and transcriptional regulators UPC2 and STP2.
Dhamgaye S, Devaux F, Manoharlal R, Vandeputte P, Shah AH, Singh A, Blugeon C, Sanglard D, Prasad R. Dhamgaye S, et al. Antimicrob Agents Chemother. 2012 Jan;56(1):495-506. doi: 10.1128/AAC.00574-11. Epub 2011 Oct 17. Antimicrob Agents Chemother. 2012. PMID: 22006003 Free PMC article. - Hsp90 governs echinocandin resistance in the pathogenic yeast Candida albicans via calcineurin.
Singh SD, Robbins N, Zaas AK, Schell WA, Perfect JR, Cowen LE. Singh SD, et al. PLoS Pathog. 2009 Jul;5(7):e1000532. doi: 10.1371/journal.ppat.1000532. Epub 2009 Jul 31. PLoS Pathog. 2009. PMID: 19649312 Free PMC article. - Candida albicans Heat Shock Proteins and Hsps-Associated Signaling Pathways as Potential Antifungal Targets.
Gong Y, Li T, Yu C, Sun S. Gong Y, et al. Front Cell Infect Microbiol. 2017 Dec 19;7:520. doi: 10.3389/fcimb.2017.00520. eCollection 2017. Front Cell Infect Microbiol. 2017. PMID: 29312897 Free PMC article. Review. - An update on antifungal targets and mechanisms of resistance in Candida albicans.
Akins RA. Akins RA. Med Mycol. 2005 Jun;43(4):285-318. doi: 10.1080/13693780500138971. Med Mycol. 2005. PMID: 16110776 Review.
Cited by
- Potent Activities of Roemerine against Candida albicans and the Underlying Mechanisms.
Ma C, Du F, Yan L, He G, He J, Wang C, Rao G, Jiang Y, Xu G. Ma C, et al. Molecules. 2015 Sep 29;20(10):17913-28. doi: 10.3390/molecules201017913. Molecules. 2015. PMID: 26426004 Free PMC article. - Antimicrobial activities and mechanisms of extract and components of herbs in East Asia.
Liang J, Huang X, Ma G. Liang J, et al. RSC Adv. 2022 Oct 12;12(45):29197-29213. doi: 10.1039/d2ra02389j. eCollection 2022 Oct 11. RSC Adv. 2022. PMID: 36320733 Free PMC article. Review. - In vitro toxicity determination of antifungal constituents from Combretum zeyheri.
Mapfunde S, Sithole S, Mukanganyama S. Mapfunde S, et al. BMC Complement Altern Med. 2016 Jun 2;16:162. doi: 10.1186/s12906-016-1150-9. BMC Complement Altern Med. 2016. PMID: 27251466 Free PMC article. - Study on the mechanisms of action of berberine combined with fluconazole against fluconazole-resistant strains of Talaromyces marneffei.
Kai-Su P, Hong L, Dong-Yan Z, Yan-Qing Z, Andrianopoulos A, Latgé JP, Cun-Wei C. Kai-Su P, et al. Front Microbiol. 2022 Nov 14;13:1033211. doi: 10.3389/fmicb.2022.1033211. eCollection 2022. Front Microbiol. 2022. PMID: 36452929 Free PMC article. - Berberine Antifungal Activity in Fluconazole-Resistant Pathogenic Yeasts: Action Mechanism Evaluated by Flow Cytometry and Biofilm Growth Inhibition in Candida spp.
da Silva AR, de Andrade Neto JB, da Silva CR, Campos Rde S, Costa Silva RA, Freitas DD, do Nascimento FB, de Andrade LN, Sampaio LS, Grangeiro TB, Magalhães HI, Cavalcanti BC, de Moraes MO, Nobre Júnior HV. da Silva AR, et al. Antimicrob Agents Chemother. 2016 May 23;60(6):3551-7. doi: 10.1128/AAC.01846-15. Print 2016 Jun. Antimicrob Agents Chemother. 2016. PMID: 27021328 Free PMC article.
References
- Hajjeh RA, Sofair AN, Harrison LH, Lyon GM, Arthington-Skaggs BA, et al. (2004) Incidence of bloodstream infections due to Candida species and in vitro susceptibilities of isolates collected from 1998 to 2000 in a population-based active surveillance program. J Clin Microbiol 42: 1519–1527. - PMC - PubMed
- Odds FC, Brown AJ, Gow NA (2003) Antifungal agents: mechanisms of action. Trends in Microbiology 11: 272–279. - PubMed
- Sucher AJ, Chahine EB, Balcer HE (2009) Echinocandins: the newest class of antifungals. Ann Pharmacother 43: 1647–57. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous