Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomised trials - PubMed (original) (raw)
Review
. 2014 Nov 29;384(9958):1929-35.
doi: 10.1016/S0140-6736(14)60584-5. Epub 2014 Aug 5.
Kennedy R Lees 2, Patrick Lyden 3, Lisa Blackwell 1, Gregory Albers 4, Erich Bluhmki 5, Thomas Brott 6, Geoff Cohen 7, Stephen Davis 8, Geoffrey Donnan 9, James Grotta 10, George Howard 11, Markku Kaste 12, Masatoshi Koga 13, Ruediger von Kummer 14, Maarten Lansberg 4, Richard I Lindley 15, Gordon Murray 7, Jean Marc Olivot 4, Mark Parsons 16, Barbara Tilley 10, Danilo Toni 17, Kazunori Toyoda 13, Nils Wahlgren 18, Joanna Wardlaw 7, William Whiteley 7, Gregory J del Zoppo 19, Colin Baigent 20, Peter Sandercock 7, Werner Hacke 21; Stroke Thrombolysis Trialists' Collaborative Group
Collaborators, Affiliations
- PMID: 25106063
- PMCID: PMC4441266
- DOI: 10.1016/S0140-6736(14)60584-5
Review
Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomised trials
Jonathan Emberson et al. Lancet. 2014.
Abstract
Background: Alteplase is effective for treatment of acute ischaemic stroke but debate continues about its use after longer times since stroke onset, in older patients, and among patients who have had the least or most severe strokes. We assessed the role of these factors in affecting good stroke outcome in patients given alteplase.
Methods: We did a pre-specified meta-analysis of individual patient data from 6756 patients in nine randomised trials comparing alteplase with placebo or open control. We included all completed randomised phase 3 trials of intravenous alteplase for treatment of acute ischaemic stroke for which data were available. Retrospective checks confirmed that no eligible trials had been omitted. We defined a good stroke outcome as no significant disability at 3-6 months, defined by a modified Rankin Score of 0 or 1. Additional outcomes included symptomatic intracranial haemorrhage (defined by type 2 parenchymal haemorrhage within 7 days and, separately, by the SITS-MOST definition of parenchymal type 2 haemorrhage within 36 h), fatal intracranial haemorrhage within 7 days, and 90-day mortality.
Findings: Alteplase increased the odds of a good stroke outcome, with earlier treatment associated with bigger proportional benefit. Treatment within 3·0 h resulted in a good outcome for 259 (32·9%) of 787 patients who received alteplase versus 176 (23·1%) of 762 who received control (OR 1·75, 95% CI 1·35-2·27); delay of greater than 3·0 h, up to 4·5 h, resulted in good outcome for 485 (35·3%) of 1375 versus 432 (30·1%) of 1437 (OR 1·26, 95% CI 1·05-1·51); and delay of more than 4·5 h resulted in good outcome for 401 (32·6%) of 1229 versus 357 (30·6%) of 1166 (OR 1·15, 95% CI 0·95-1·40). Proportional treatment benefits were similar irrespective of age or stroke severity. Alteplase significantly increased the odds of symptomatic intracranial haemorrhage (type 2 parenchymal haemorrhage definition 231 [6·8%] of 3391 vs 44 [1·3%] of 3365, OR 5·55, 95% CI 4·01-7·70, p<0·0001; SITS-MOST definition 124 [3·7%] vs 19 [0·6%], OR 6·67, 95% CI 4·11-10·84, p<0·0001) and of fatal intracranial haemorrhage within 7 days (91 [2·7%] vs 13 [0·4%]; OR 7·14, 95% CI 3·98-12·79, p<0·0001). The relative increase in fatal intracranial haemorrhage from alteplase was similar irrespective of treatment delay, age, or stroke severity, but the absolute excess risk attributable to alteplase was bigger among patients who had more severe strokes. There was no excess in other early causes of death and no significant effect on later causes of death. Consequently, mortality at 90 days was 608 (17·9%) in the alteplase group versus 556 (16·5%) in the control group (hazard ratio 1·11, 95% CI 0·99-1·25, p=0·07). Taken together, therefore, despite an average absolute increased risk of early death from intracranial haemorrhage of about 2%, by 3-6 months this risk was offset by an average absolute increase in disability-free survival of about 10% for patients treated within 3·0 h and about 5% for patients treated after 3·0 h, up to 4·5 h.
Interpretation: Irrespective of age or stroke severity, and despite an increased risk of fatal intracranial haemorrhage during the first few days after treatment, alteplase significantly improves the overall odds of a good stroke outcome when delivered within 4·5 h of stroke onset, with earlier treatment associated with bigger proportional benefits.
Funding: UK Medical Research Council, British Heart Foundation, University of Glasgow, University of Edinburgh.
Copyright © 2014 Emberson et al. Open Access article distributed under the terms of CC BY. Published by Elsevier Ltd. All rights reserved.
Figures
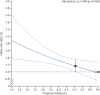
Figure 1
Effect of timing of alteplase treatment on good stroke outcome (mRS 0–1) The solid line is the best linear fit between the log odds ratio for a good stroke outcome for patients given alteplase compared with those given control (vertical axis) and treatment delay (horizontal axis; pinteraction=0·016). Estimates are derived from a regression model in which alteplase, time to treatment, age, and stroke severity (handled in a quadratic manner) are included as main effects but the only treatment interaction included is with time to treatment. Only 198 patients (159 from IST–3) had a time from stroke onset to treatment of more than 6 h. The white box shows the point at which the estimated treatment effect crosses 1. The black box shows the point at which the lower 95% CI for the estimated treatment effect first crosses 1·0. mRS=modified Rankin Scale.
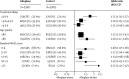
Figure 2
Effect of alteplase on good stroke outcome (mRS 0–1), by treatment delay, age, and stroke severity *For each of the three baseline characteristics, estimates were derived from a single logistic regression model stratified by trial, which enables separate estimation of the OR for each subgroup after adjustment for the other two baseline characteristics (but not for possible interactions with those characteristics). mRS=modified Rankin Scale.
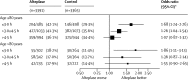
Figure 3
Effect of alteplase on a good stroke outcome (mRS 0–1) by age, with different treatment delays Effect of age on the interaction between treatment delay and treatment effect p=0·08 (ie, not significant but, if anything, in the direction of it lengthening, not shortening, the period during which alteplase is effective in older people). *All six estimates derived from a single stratified logistic regression model that enables the odds ratio to be estimated separately for each group (also adjusted for baseline National Institutes of Health Stroke Scale score). mRS=modified Rankin Scale.
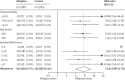
Figure 4
Effect of alteplase on fatal intracranial haemorrhage within 7 days by treatment delay, age, and stroke severity *For each of the three baseline characteristics, estimates were derived from a single logistic regression model stratified by trial, which enables separate estimation of the OR for each subgroup after adjustment for the other two baseline characteristics (but not possible interactions with those characteristics). The overall effect in all patients is the trial-stratified logistic regression estimate adjusted only for treatment allocation. NE=not estimable.
Figure 5
Effect of alteplase on 90-day mortality by follow-up period Patients can only contribute to a particular risk period if they have already survived any preceding periods. *Estimated by Cox proportional hazards regression stratified by trial (and adjusted only for treatment allocation). †Includes 91 versus 13 deaths caused by intracranial haemorrhage (with evidence of parenchymal haemorrhage type 2; figure 4) and 191 versus 191 deaths from other causes.
Figure 6
Effect of alteplase on 90-day mortality by treatment delay *Estimated by Cox proportional hazards regression stratified by trial (and adjusted only for treatment allocation). HR=hazard ratio.
Comment in
- Alteplase in acute ischaemic stroke: the need for speed.
Hill MD, Coutts SB. Hill MD, et al. Lancet. 2014 Nov 29;384(9958):1904-6. doi: 10.1016/S0140-6736(14)60662-0. Epub 2014 Aug 5. Lancet. 2014. PMID: 25106064 No abstract available. - Pooled RCTs: alteplase within 4.5 hours of ischemic stroke improves the likelihood of good outcome.
Uchino K. Uchino K. Ann Intern Med. 2015 Jan 20;162(2):JC3. doi: 10.7326/ACPJC-2015-162-2-003. Ann Intern Med. 2015. PMID: 25599363 No abstract available. - Timing of thrombolysis for acute ischaemic stroke: the earlier the treatment the better the outcome, irrespective of age or stroke severity.
Lorenzano S. Lorenzano S. Evid Based Med. 2015 Jun;20(3):108. doi: 10.1136/ebmed-2014-110155. Epub 2015 Apr 8. Evid Based Med. 2015. PMID: 25854628 No abstract available. - Thrombolysis for stroke: clinical judgment at its apogee.
[No authors listed] [No authors listed] Lancet. 2015 Apr 11;385(9976):1366. doi: 10.1016/S0140-6736(15)60702-4. Lancet. 2015. PMID: 25890403 No abstract available. - Thrombolysis in acute stroke.
Brunström M, Carlberg B. Brunström M, et al. Lancet. 2015 Apr 11;385(9976):1394-5. doi: 10.1016/S0140-6736(15)60715-2. Lancet. 2015. PMID: 25890416 No abstract available. - Thrombolysis in acute stroke.
Appelros P, Terént A. Appelros P, et al. Lancet. 2015 Apr 11;385(9976):1394. doi: 10.1016/S0140-6736(15)60714-0. Lancet. 2015. PMID: 25890417 No abstract available. - Thrombolysis in acute stroke.
Newman DH. Newman DH. Lancet. 2015 Apr 11;385(9976):1395. doi: 10.1016/S0140-6736(15)60716-4. Lancet. 2015. PMID: 25890418 No abstract available. - Thrombolysis in acute stroke.
Caso V, Michel P. Caso V, et al. Lancet. 2015 Apr 11;385(9976):1395-6. doi: 10.1016/S0140-6736(15)60717-6. Lancet. 2015. PMID: 25890419 No abstract available. - Thrombolysis in acute stroke--authors' reply.
Emberson J, Lees KR, Lyden P, Baigent C, Sandercock P, Hacke W; Stroke Treatment Trialists' Collaboration. Emberson J, et al. Lancet. 2015 Apr 11;385(9976):1396. doi: 10.1016/S0140-6736(15)60718-8. Lancet. 2015. PMID: 25890422 No abstract available.
Similar articles
- Risk of intracerebral haemorrhage with alteplase after acute ischaemic stroke: a secondary analysis of an individual patient data meta-analysis.
Whiteley WN, Emberson J, Lees KR, Blackwell L, Albers G, Bluhmki E, Brott T, Cohen G, Davis S, Donnan G, Grotta J, Howard G, Kaste M, Koga M, von Kummer R, Lansberg MG, Lindley RI, Lyden P, Olivot JM, Parsons M, Toni D, Toyoda K, Wahlgren N, Wardlaw J, Del Zoppo GJ, Sandercock P, Hacke W, Baigent C; Stroke Thrombolysis Trialists' Collaboration. Whiteley WN, et al. Lancet Neurol. 2016 Aug;15(9):925-933. doi: 10.1016/S1474-4422(16)30076-X. Epub 2016 Jun 8. Lancet Neurol. 2016. PMID: 27289487 - Early administration of aspirin in patients treated with alteplase for acute ischaemic stroke: a randomised controlled trial.
Zinkstok SM, Roos YB; ARTIS investigators. Zinkstok SM, et al. Lancet. 2012 Aug 25;380(9843):731-7. doi: 10.1016/S0140-6736(12)60949-0. Epub 2012 Jun 28. Lancet. 2012. PMID: 22748820 Clinical Trial. - Time to treatment with intravenous alteplase and outcome in stroke: an updated pooled analysis of ECASS, ATLANTIS, NINDS, and EPITHET trials.
Lees KR, Bluhmki E, von Kummer R, Brott TG, Toni D, Grotta JC, Albers GW, Kaste M, Marler JR, Hamilton SA, Tilley BC, Davis SM, Donnan GA, Hacke W; ECASS, ATLANTIS, NINDS and EPITHET rt-PA Study Group; Allen K, Mau J, Meier D, del Zoppo G, De Silva DA, Butcher KS, Parsons MW, Barber PA, Levi C, Bladin C, Byrnes G. Lees KR, et al. Lancet. 2010 May 15;375(9727):1695-703. doi: 10.1016/S0140-6736(10)60491-6. Lancet. 2010. PMID: 20472172 - Thrombolysis for acute ischaemic stroke.
Wardlaw JM, Murray V, Berge E, del Zoppo GJ. Wardlaw JM, et al. Cochrane Database Syst Rev. 2014 Jul 29;2014(7):CD000213. doi: 10.1002/14651858.CD000213.pub3. Cochrane Database Syst Rev. 2014. PMID: 25072528 Free PMC article. Review. - Intravenous alteplase for stroke with unknown time of onset guided by advanced imaging: systematic review and meta-analysis of individual patient data.
Thomalla G, Boutitie F, Ma H, Koga M, Ringleb P, Schwamm LH, Wu O, Bendszus M, Bladin CF, Campbell BCV, Cheng B, Churilov L, Ebinger M, Endres M, Fiebach JB, Fukuda-Doi M, Inoue M, Kleinig TJ, Latour LL, Lemmens R, Levi CR, Leys D, Miwa K, Molina CA, Muir KW, Nighoghossian N, Parsons MW, Pedraza S, Schellinger PD, Schwab S, Simonsen CZ, Song SS, Thijs V, Toni D, Hsu CY, Wahlgren N, Yamamoto H, Yassi N, Yoshimura S, Warach S, Hacke W, Toyoda K, Donnan GA, Davis SM, Gerloff C; Evaluation of unknown Onset Stroke thrombolysis trials (EOS) investigators. Thomalla G, et al. Lancet. 2020 Nov 14;396(10262):1574-1584. doi: 10.1016/S0140-6736(20)32163-2. Epub 2020 Nov 8. Lancet. 2020. PMID: 33176180 Free PMC article.
Cited by
- Acupuncture and its role in the treatment of ischemic stroke: A review.
Wang Z, Wang M, Zhao H. Wang Z, et al. Medicine (Baltimore). 2024 Oct 4;103(40):e39820. doi: 10.1097/MD.0000000000039820. Medicine (Baltimore). 2024. PMID: 39465714 Free PMC article. Review. - A Neurodisparity Index of Nationwide Access to Neurological Health Care in Northern Ireland.
McCarron MO, Clarke M, Burns P, McCormick M, McCarron P, Forbes RB, McCarron LV, Mullan F, McVerry F. McCarron MO, et al. Front Neurol. 2021 Feb 12;12:608070. doi: 10.3389/fneur.2021.608070. eCollection 2021. Front Neurol. 2021. PMID: 33643193 Free PMC article. - Thrombectomy in large vessel occlusion stroke-Does age matter?
Rezai MK, Dalen I, Advani R, Fjetland L, Kurz KD, Sandve KO, Kurz MW. Rezai MK, et al. Acta Neurol Scand. 2022 Nov;146(5):628-634. doi: 10.1111/ane.13691. Epub 2022 Aug 27. Acta Neurol Scand. 2022. PMID: 36029034 Free PMC article. - Influence Factors and Predictive Models for the Outcome of Patients with Ischemic Stroke after Intravenous Thrombolysis: A Multicenter Retrospective Cohort Study.
Hu J, Fang Z, Lu X, Wang F, Zhang N, Pan W, Fu X, Huang G, Tan X, Chen W. Hu J, et al. Oxid Med Cell Longev. 2022 Aug 17;2022:3363735. doi: 10.1155/2022/3363735. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 36035225 Free PMC article. Retracted. - Trends in Stroke Thrombolysis Care Metrics and Outcomes by Race and Ethnicity, 2003-2021.
Man S, Solomon N, Mac Grory B, Alhanti B, Saver JL, Smith EE, Xian Y, Bhatt DL, Schwamm LH, Uchino K, Fonarow GC. Man S, et al. JAMA Netw Open. 2024 Feb 5;7(2):e2352927. doi: 10.1001/jamanetworkopen.2023.52927. JAMA Netw Open. 2024. PMID: 38324315 Free PMC article.
References
- Hacke W, Donnan G, Fieschi C, the ATLANTIS Trials Investigators. the ECASS Trials Investigators. the NINDS rt-PA Study Group Investigators Association of outcome with early stroke treatment: pooled analysis of ATLANTIS, ECASS, and NINDS rt-PA stroke trials. Lancet. 2004;363:768–774. - PubMed
- Lees KR, Bluhmki E, von Kummer R, for the ECASS. ATLANTIS. NINDS and EPITHET rt-PA Study Group Investigators Time to treatment with intravenous alteplase and outcome in stroke: an updated pooled analysis of ECASS, ATLANTIS, NINDS, and EPITHET trials. Lancet. 2010;375:1695–1703. - PubMed
- European Stroke Organisation (ESO) Executive Committee. the ESO Writing Committee Guidelines for management of ischaemic stroke and transient ischaemic attack 2008. Cerebrovasc Dis. 2008;25:457–507. - PubMed
- European Stroke Organisation guidelines for stroke management: Update January 2009. Available from: http://www.eso-stroke.org/eso-stroke/education/education... (accessed March 3, 2014).
- Minematsu K, Toyoda K, Hirano T. Guidelines for the intravenous application of recombinant tissue-type plasminogen activator (alteplase), the second edition, October 2012: a guideline from the Japan Stroke Society. J Stroke Cerebrovasc. 2013;22:571–600. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS038710/NS/NINDS NIH HHS/United States
- G0400069/MRC_/Medical Research Council/United Kingdom
- BHF_/British Heart Foundation/United Kingdom
- MR/K026992/1/MRC_/Medical Research Council/United Kingdom
- MC_U137686849/MRC_/Medical Research Council/United Kingdom
- G0902303/MRC_/Medical Research Council/United Kingdom
- R01 NS053716/NS/NINDS NIH HHS/United States
- G0800803/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical