Twenty year experience of the oral rabies vaccine SAG2 in wildlife: a global review - PubMed (original) (raw)
Review
Twenty year experience of the oral rabies vaccine SAG2 in wildlife: a global review
Philippe Mähl et al. Vet Res. 2014.
Abstract
The SAG2 vaccine (RABIGEN® SAG2) is a modified live attenuated rabies virus vaccine, selected from the SAD Bern strain in a two-step process of amino acid mutation using neutralizing monoclonal antibodies. The strain is genetically stable and does not spread in vivo or induce a persistent infection. Its absence of residual pathogenicity was extensively demonstrated in multiple target and non target species (such as wild carnivores and rodent species), including non-human primates. The efficacy of SAG2 baits was demonstrated according to the EU requirements for the red fox and raccoon dog. The use of safe and potent rabies vaccines such as SAG2 largely contributed to the elimination of rabies in Estonia, France, Italy and Switzerland. Importantly, these countries were declared free of rabies after few years of oral vaccination campaigns with SAG2 baits distributed with an appropriate strategy. The excellent tolerance of the SAG2 vaccine has been confirmed in the field since its first use in 1993. No safety issues have been reported, and in particular no vaccine-induced rabies cases were diagnosed, after the distribution of more than 20 million SAG2 baits in Europe.
Figures
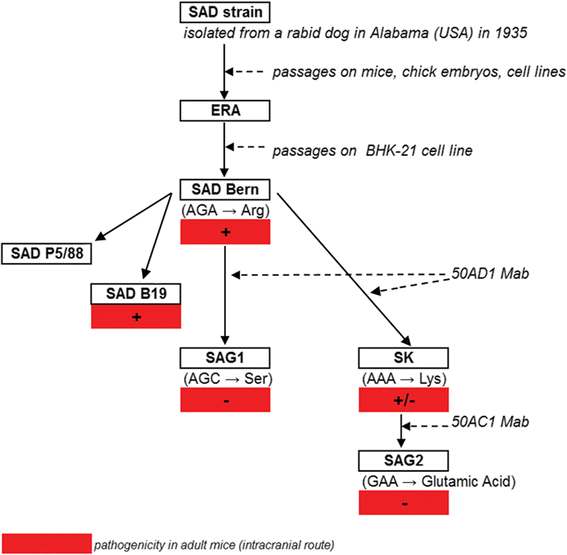
Figure 1
Construction of the SAG2 strain. ERA = Evelyn Rokitnicki Abelseth, Mab = monoclonal antibody. The parental SAD strain was isolated from the salivary glands of a rabid dog in the USA during 1935, which was passaged in mice, chick embryos, and various cell lines and was re-named ERA (Evelyn Rokitnicki Abelseth). The SAD Bern strain is a cell-adapted derivative of the ERA strain. The SAD Bern strain was cultivated in the presence of monoclonal antibodies binding specifically to one of the two major antigenic sites (antigenic site III) of the rabies virus glycoprotein, involved in virus pathogenicity. Under the selective pressure of these monoclonal antibodies, only variants of SAD Bern bearing an amino-acid substitution at the critical position 333 of the rabies virus glycoprotein escaped neutralisation in culture. An avirulent mutant, SAG1 (for SAD Avirulent Gif), in which arginine at position 333 was substituted by serine, was isolated from SAD Bern with monoclonal antibody (Mab) 50 AD1. The SAG2 strain was constructed from SAD Bern in a two-step selection procedure using neutralizing monoclonal antibodies. First, a mutant strain (SK) was selected from SAD Bern, where the arginine at position 333 was replaced by lysine. SAG2, a non pathogenic mutant resistant to neutralisation by monoclonal antibody 50 AC1 was selected from SK, where the lysine at position 333 was replaced by a glutamic acid. Thus, SAG2 can be considered as a double avirulent mutant, since the codon GAA, which codes for glutamic acid, differs from the codon AGA from SAD Bern (coding for arginine) by two nucleotides.
Figure 2
SAG2 Vaccine bait. This figure shows the bait, the PVC/aluminium blister containing the liquid suspension of the SAG2 strain. SAG2 bait and the blister are both labelled with “Rabies vaccine, do not touch”.
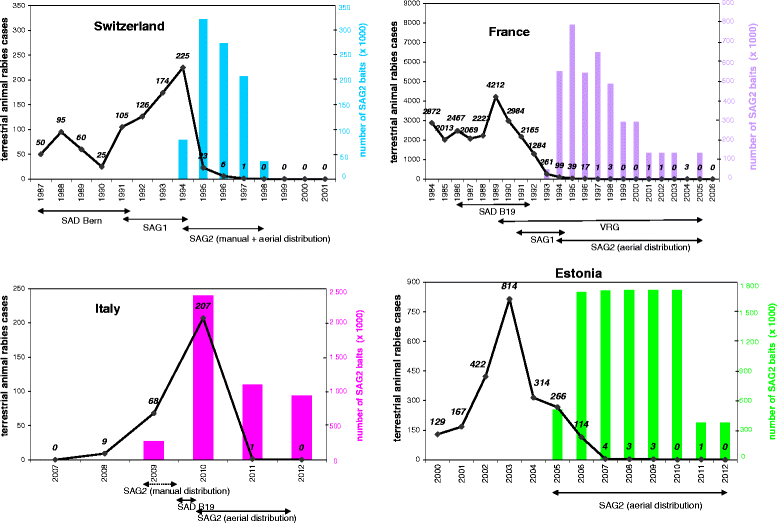
Figure 3
Animal rabies cases before and after vaccination campaigns with SAG2 in Switzerland, France, Estonia, Italy. The impact of the vaccination with SAG2 baits on the number of terrestrial animal rabies cases is illustrated in four countries, Switzerland, France, Estonia and Italy.
Similar articles
- Genetic strain modification of a live rabies virus vaccine widely used in Europe for wildlife oral vaccination.
Cliquet F, Robardet E, Picard Meyer E. Cliquet F, et al. Antiviral Res. 2013 Oct;100(1):84-9. doi: 10.1016/j.antiviral.2013.07.012. Epub 2013 Jul 27. Antiviral Res. 2013. PMID: 23899697 - Safety and efficacy of the oral rabies vaccine SAG2 in raccoon dogs.
Cliquet F, Guiot AL, Munier M, Bailly J, Rupprecht CE, Barrat J. Cliquet F, et al. Vaccine. 2006 May 15;24(20):4386-92. doi: 10.1016/j.vaccine.2006.02.057. Epub 2006 Mar 24. Vaccine. 2006. PMID: 16603277 - Efficacy of three oral rabies vaccine-baits in the red fox: a comparison.
Artois M, Masson E, Barrat J, Aubert MF. Artois M, et al. Vet Microbiol. 1993 Dec;38(1-2):167-72. doi: 10.1016/0378-1135(93)90083-j. Vet Microbiol. 1993. PMID: 8128598 - Rabies in Estonia: situation before and after the first campaigns of oral vaccination of wildlife with SAG2 vaccine bait.
Niin E, Laine M, Guiot AL, Demerson JM, Cliquet F. Niin E, et al. Vaccine. 2008 Jul 4;26(29-30):3556-65. doi: 10.1016/j.vaccine.2008.04.056. Epub 2008 May 12. Vaccine. 2008. PMID: 18524435 Review. - Elimination of terrestrial rabies in Western European countries.
Cliquet F, Aubert M. Cliquet F, et al. Dev Biol (Basel). 2004;119:185-204. Dev Biol (Basel). 2004. PMID: 15747421 Review.
Cited by
- Efficient distribution of oral vaccines examined by infrared triggered camera for advancing the control of raccoon dog rabies in South Korea.
Cho HK, Shin YJ, Shin NS, Chae JS. Cho HK, et al. J Vet Med Sci. 2020 Dec 5;82(11):1685-1692. doi: 10.1292/jvms.20-0173. Epub 2020 Oct 8. J Vet Med Sci. 2020. PMID: 33028748 Free PMC article. - Oral bait handout as a method to access roaming dogs for rabies vaccination in Goa, India: A proof of principle study.
Gibson AD, Yale G, Vos A, Corfmat J, Airikkala-Otter I, King A, Wallace RM, Gamble L, Handel IG, Mellanby RJ, Bronsvoort BMC, Mazeri S. Gibson AD, et al. Vaccine X. 2019 Mar 1;1:100015. doi: 10.1016/j.jvacx.2019.100015. eCollection 2019 Apr 11. Vaccine X. 2019. PMID: 31384737 Free PMC article. - Review of Oral Rabies Vaccination of Dogs and Its Application in India.
Yale G, Lopes M, Isloor S, Head JR, Mazeri S, Gamble L, Dukpa K, Gongal G, Gibson AD. Yale G, et al. Viruses. 2022 Jan 14;14(1):155. doi: 10.3390/v14010155. Viruses. 2022. PMID: 35062358 Free PMC article. Review. - Self-disseminating vaccines for emerging infectious diseases.
Murphy AA, Redwood AJ, Jarvis MA. Murphy AA, et al. Expert Rev Vaccines. 2016;15(1):31-9. doi: 10.1586/14760584.2016.1106942. Epub 2015 Nov 2. Expert Rev Vaccines. 2016. PMID: 26524478 Free PMC article. Review. - The Presence of Rabies Virus-Neutralizing Antibody in Wild Boars (Sus scrofa), a Non-Target Bait Vaccine Animal in Korea.
Kim HH, Yang DK, Wang JY, An DJ. Kim HH, et al. Vet Sci. 2020 Jul 10;7(3):90. doi: 10.3390/vetsci7030090. Vet Sci. 2020. PMID: 32664240 Free PMC article.
References
- Rupprecht CE, Shlim DR. Rabies. Atlanta: CDC; 2014. Infectious diseases related to travel.
- WHO: WHO expert consultation on rabies, 1streport. In WHO Techn Rep Series, Volume 931. Geneva: World Health Organization; 2005:121. - PubMed
- WHO . Report of WHO consultation on requirements and criteria for field trials on oral rabies vaccination of dogs and wild carnivores: 1-2 March 1989. Geneva: World Health Organization; 1989. pp. 1–15.
- WHO: WHO expert committee on rabies, 8threport. In WHO Techn Rep Series, Volume 824. Geneva: World Health Organization; 1992:84. - PubMed
- WHO . Report of the fourth WHO consultation on oral immunization of dogs against rabies: 14-15 June 1993. Geneva: World Health Organization; 1993. pp. 1–17.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical