A new pathway for mitochondrial quality control: mitochondrial-derived vesicles - PubMed (original) (raw)
Review
A new pathway for mitochondrial quality control: mitochondrial-derived vesicles
Ayumu Sugiura et al. EMBO J. 2014.
Abstract
The last decade has been marked by tremendous progress in our understanding of the cell biology of mitochondria, with the identification of molecules and mechanisms that regulate their fusion, fission, motility, and the architectural transitions within the inner membrane. More importantly, the manipulation of these machineries in tissues has provided links between mitochondrial dynamics and physiology. Indeed, just as the proteins required for fusion and fission were identified, they were quickly linked to both rare and common human diseases. This highlighted the critical importance of this emerging field to medicine, with new hopes of finding drugable targets for numerous pathologies, from neurodegenerative diseases to inflammation and cancer. In the midst of these exciting new discoveries, an unexpected new aspect of mitochondrial cell biology has been uncovered; the generation of small vesicular carriers that transport mitochondrial proteins and lipids to other intracellular organelles. These mitochondrial-derived vesicles (MDVs) were first found to transport a mitochondrial outer membrane protein MAPL to a subpopulation of peroxisomes. However, other MDVs did not target peroxisomes and instead fused with the late endosome, or multivesicular body. The Parkinson's disease-associated proteins Vps35, Parkin, and PINK1 are involved in the biogenesis of a subset of these MDVs, linking this novel trafficking pathway to human disease. In this review, we outline what has been learned about the mechanisms and functional importance of MDV transport and speculate on the greater impact of these pathways in cellular physiology.
Keywords: PINK1; Parkin; mitochondria; quality control; vesicle transport.
© 2014 The Authors.
Figures
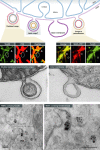
Figure 1. Summary of MDVs cargo variability
Immunofluorescent and EM images illustrate the diversity of cargo-selected MDVs. Immunofluorescent staining of Tom20 (an outer membrane protein) and pyruvate dehydrogenase (PDH, matrix protein) reveals a number of cargo-selected vesicular structures lying outside of the mitochondria (top left panels, circles versus arrowheads). Although Tom20 is absent from PDH-positive structures (arrowheads), EM and biochemical experiments confirm that these vesicles are double membrane bound. An example is shown to the left where both membranes are seen within the vesicle emerging from the intact mitochondria [with permission from Soubannier et al ( 2012b)]. Similar cargo selectivity is seen for MDVs carrying MAPL that target the peroxisomes [top right panel of immunofluorescent images, taken with permission from Neuspiel et al ( 2008)]. We also observe single membrane MDVs derived from just the outer mitochondrial membrane (EM panel on right side). Bottom electron microscopic pictures show MDVs containing Tom20 labeled by immunogold particles enter the multivesicular body [taken with permission from Soubannier et al ( 2012a)]. Scale bars in EM pictures represent 100 nm.
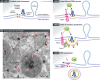
Figure 2. Working hypothesis for vesicle initiation by PINK1 and Parkin
(A) Immunogold staining of endogenous Tom20 within COS7 cells reveals the regular spacing of the import channels indicated by arrowheads. Note the close tethering of three multivesicular bodies to the mitochondria. (B) An illustration of our working hypothesis of PINK1/Parkin-mediated MDV formation. In Step 1, unfolded, oxidized proteins within matrix, triggered by ROS or failure to assemble, leads to protein aggregation (blue). Oxidation of cardiolipin will generate PA, contributing to altered membrane curvature. In Step 2, protein aggregates may saturate chaperones, leading to a very localized failure to import at an individual channel. In addition, local oxidation of cardiolipin would further interfere with import channels. PINK1, which is rapidly imported, would then accumulate at these failed import channels. In Step 3, PINK1 phosphorylates both ubiquitin and the ubiquitin-like domain of Parkin, stabilizing the recruitment of activated Parkin. The ubiquitination activity of Parkin is required to generate MDVs, suggesting that domains on the surface may be cleared. In Step 4, a vesicle is formed and released in a process that will certainly involve a number of unidentified proteins. Future studies are needed to test this hypothesis and uncover the details governing the generation of MDVs.
Figure 3. Outline of the 4 pathways of mitochondrial quality control
A schematic diagram depicting the presence of mitochondrial proteases within the mitochondrial matrix and intermembrane space, which likely acts as a first line of defense against unfolded and oxidized soluble proteins. Outer membrane proteins are instead removed from the mitochondria through a retrotranslocation pathway following ubiquitination. Degradation of these proteins is completed within the cytosolic proteasome, similar to the ER-associated degradation pathway. We propose that the third line of defense is the removal of mitochondrial patches through the generation of MDVs, which transit to the late endosome. Only upon complete mitochondrial dysfunction, or upon a failure of most import channels would the entire organelle be targeted to the autophagosome. Different tissues and cellular states may rely on each of these mechanisms to a variable degree, making it important to understand the levels of redundancy and overlap among these pathways.
Similar articles
- Cargo-selected transport from the mitochondria to peroxisomes is mediated by vesicular carriers.
Neuspiel M, Schauss AC, Braschi E, Zunino R, Rippstein P, Rachubinski RA, Andrade-Navarro MA, McBride HM. Neuspiel M, et al. Curr Biol. 2008 Jan 22;18(2):102-8. doi: 10.1016/j.cub.2007.12.038. Curr Biol. 2008. PMID: 18207745 - Vps35 mediates vesicle transport between the mitochondria and peroxisomes.
Braschi E, Goyon V, Zunino R, Mohanty A, Xu L, McBride HM. Braschi E, et al. Curr Biol. 2010 Jul 27;20(14):1310-5. doi: 10.1016/j.cub.2010.05.066. Epub 2010 Jul 8. Curr Biol. 2010. PMID: 20619655 - Parkin and PINK1 function in a vesicular trafficking pathway regulating mitochondrial quality control.
McLelland GL, Soubannier V, Chen CX, McBride HM, Fon EA. McLelland GL, et al. EMBO J. 2014 Feb 18;33(4):282-95. doi: 10.1002/embj.201385902. Epub 2014 Jan 20. EMBO J. 2014. PMID: 24446486 Free PMC article. - Mitochondrial-derived vesicles: Recent insights.
Popov LD. Popov LD. J Cell Mol Med. 2022 Jun;26(12):3323-3328. doi: 10.1111/jcmm.17391. Epub 2022 May 18. J Cell Mol Med. 2022. PMID: 35582908 Free PMC article. Review. - Mitochondrial vesicles: an ancient process providing new links to peroxisomes.
Andrade-Navarro MA, Sanchez-Pulido L, McBride HM. Andrade-Navarro MA, et al. Curr Opin Cell Biol. 2009 Aug;21(4):560-7. doi: 10.1016/j.ceb.2009.04.005. Epub 2009 May 5. Curr Opin Cell Biol. 2009. PMID: 19423315 Review.
Cited by
- Open questions: seeking a holistic approach for mitochondrial research.
McBride HM. McBride HM. BMC Biol. 2015 Feb 5;13:8. doi: 10.1186/s12915-015-0120-x. BMC Biol. 2015. PMID: 25651813 Free PMC article. - Rapid parallel measurements of macroautophagy and mitophagy in mammalian cells using a single fluorescent biosensor.
Sargsyan A, Cai J, Fandino LB, Labasky ME, Forostyan T, Colosimo LK, Thompson SJ, Graham TE. Sargsyan A, et al. Sci Rep. 2015 Jul 28;5:12397. doi: 10.1038/srep12397. Sci Rep. 2015. PMID: 26215030 Free PMC article. - Stable heteroplasmy at the single-cell level is facilitated by intercellular exchange of mtDNA.
Jayaprakash AD, Benson EK, Gone S, Liang R, Shim J, Lambertini L, Toloue MM, Wigler M, Aaronson SA, Sachidanandam R. Jayaprakash AD, et al. Nucleic Acids Res. 2015 Feb 27;43(4):2177-87. doi: 10.1093/nar/gkv052. Epub 2015 Feb 4. Nucleic Acids Res. 2015. PMID: 25653158 Free PMC article. - NSP4 and ORF9b of SARS-CoV-2 Induce Pro-Inflammatory Mitochondrial DNA Release in Inner Membrane-Derived Vesicles.
Faizan MI, Chaudhuri R, Sagar S, Albogami S, Chaudhary N, Azmi I, Akhtar A, Ali SM, Kumar R, Iqbal J, Joshi MC, Kharya G, Seth P, Roy SS, Ahmad T. Faizan MI, et al. Cells. 2022 Sep 23;11(19):2969. doi: 10.3390/cells11192969. Cells. 2022. PMID: 36230930 Free PMC article. - Mitochondria-derived vesicles: potential nano-batteries to recharge the cellular powerhouse.
Mishra S, Deep G. Mishra S, et al. Extracell Vesicles Circ Nucl Acids. 2024 Jun;5(2):271-275. doi: 10.20517/evcna.2023.71. Epub 2024 Jun 10. Extracell Vesicles Circ Nucl Acids. 2024. PMID: 39092319 Free PMC article.
References
- Andrade-Navarro MA, Sanchez-Pulido L, McBride HM. Mitochondrial vesicles: an ancient process providing new links to peroxisomes. Curr Opin Cell Biol. 2009;21:560–567. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous