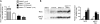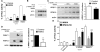WNK-SPAK-NCC cascade revisited: WNK1 stimulates the activity of the Na-Cl cotransporter via SPAK, an effect antagonized by WNK4 - PubMed (original) (raw)
. 2014 Nov;64(5):1047-53.
doi: 10.1161/HYPERTENSIONAHA.114.04036. Epub 2014 Aug 11.
Chong Zhang 1, Christelle Soukaseum 1, Erika Moreno 1, Diana Pacheco-Alvarez 1, Emmanuelle Vidal-Petiot 1, María Castañeda-Bueno 1, Norma Vázquez 1, Lorena Rojas-Vega 1, Nicholas P Meermeier 1, Shaunessy Rogers 1, Xavier Jeunemaitre 1, Chao-Ling Yang 1, David H Ellison 1, Gerardo Gamba 2, Juliette Hadchouel 2
Affiliations
- PMID: 25113964
- PMCID: PMC5832045
- DOI: 10.1161/HYPERTENSIONAHA.114.04036
WNK-SPAK-NCC cascade revisited: WNK1 stimulates the activity of the Na-Cl cotransporter via SPAK, an effect antagonized by WNK4
María Chávez-Canales et al. Hypertension. 2014 Nov.
Abstract
The with-no-lysine (K) kinases, WNK1 and WNK4, are key regulators of blood pressure. Their mutations lead to familial hyperkalemic hypertension (FHHt), associated with an activation of the Na-Cl cotransporter (NCC). Although it is clear that WNK4 mutants activate NCC via Ste20 proline-alanine-rich kinase, the mechanisms responsible for WNK1-related FHHt and alterations in NCC activity are not as clear. We tested whether WNK1 modulates NCC through WNK4, as predicted by some models, by crossing our recently developed WNK1-FHHt mice (WNK1(+/FHHt)) with WNK4(-/-) mice. Surprisingly, the activated NCC, hypertension, and hyperkalemia of WNK1(+/FHHt) mice remain in the absence of WNK4. We demonstrate that WNK1 powerfully stimulates NCC in a WNK4-independent and Ste20 proline-alanine-rich kinase-dependent manner. Moreover, WNK4 decreases the WNK1 and WNK3-mediated activation of NCC. Finally, the formation of oligomers of WNK kinases through their C-terminal coiled-coil domain is essential for their activity toward NCC. In conclusion, WNK kinases form a network in which WNK4 associates with WNK1 and WNK3 to regulate NCC.
Keywords: Xenopus laevis; familial hypertensive hyperkalemia; hypertension, renal; kidney tubules, distal; mice, knockout; pseudohypoaldosteronism, type II; water-electrolyte balance.
© 2014 American Heart Association, Inc.
Conflict of interest statement
Conflict(s) of Interest/Disclosure(s). None
Figures
Figure 1. L-WNK1 induces hyperkalemic hypertension and NCC phosphorylation independently of WNK4 in mice
(A) Renin transcripts level was measured by quantitative real-time PCR in the kidney cortex of _WNK4_−/− (n=4), WNK1+/i1lox (n=5), WNK1+/FHHt (n=6) and _WNK1+/FHHt:WNK4_−/− (n=3) males. Results are expressed in arbitrary units relative to the expression of ubc. The control group (WNK1+/i1lox mice) has been arbitrarily set to 1. Data are mean ± s.e.m. *: P<0.05 compared to the control group. (B) Left: Immunoblots performed on membrane-enriched fraction of renal cortex dissected from _WNK4_−/− (n=4), WNK1+/i1lox (n=6), WNK1+/FHHt (n=6) and _WNK1+/FHHt:WNK4_−/− (n=5) males, incubated with antibodies against anti-NCC or its T53 phosphorylated residue (pNCC-T53). Right: Densitometric analysis of the immunoblots. Data, expressed as a percentage of the average signal intensity measured in the control group, set arbitrarily to 100%, are mean ± s.e.m. *: P<0.05, **: P<0.01 and ***: P<0.001 compared to the control group (Student’s unpaired t-test).
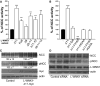
Figure 2. L-WNK1 is a powerful activator of NCC activity in Xenopus laevis oocytes
(A) NCC activity was measured in Xenopus oocytes overexpressing NCC alone or in combination with different L-WNK1 splicing variants. All L-WNK1 variants, except the one deleted from exon 9 (L-WNK1-Δ9), stimulated NCC activity. (B) Effect of L-WNK1-Δ11 point mutations affecting its kinase activity (Δ11-DA), SPAK binding (Δ11-F316A), dimer formation (Δ11-HQ) or both (Δ11-F316A-HQ) on NCC activity. For (A) and (B), NCC activity in oocytes injected with NCC cDNA alone (black bar) was arbitrarily set to 100%. Data are mean ± s.e.m. ***: P<0.001 and ****: P<0.0001 vs. NCC alone (Student’s unpaired t-test). (C) Immunoblots from NCC-HEK293 cells transiently transfected with NCC and human L-WNK1-Δ11-myc constructs, and probed with antibodies indicated. Data, indicated below the blots, are mean ± s.e.m. **: P<0.01 and ***: P<0.001 vs. the control (Student’s unpaired t-test). (D) The inhibition of L-WNK1 expression by RNA-interference decreased NCC phosphorylation. Immunoblots from NCC-HEK-293 cells transfected with control and WNK1-specific siRNA, probed with the antibodies indicated.
Figure 3. WNK4 inhibits the activation of NCC by L-WNK1 and WNK3
(A) NCC activity was measured in Xenopus oocytes injected alone or in combination with L-WNK1-Δ11 (WNK1), WNK3 and/or WNK4 as stated. WNK4 not only inhibited NCC activity but also prevented the L-WNK1-Δ11 or WNK3-dependent stimulation of NCC (N=15 independent experiments per combination). (B) Immunoblots from NCC-HEK293 cells transiently overexpressing L-WNK1-Δ11 and WNK4. WNK4 overexpression prevented the activation of NCC by L-WNK1-Δ11. (C-D) Immunoblots from NCC HEK293 cells transiently transfected with myc-tagged L-WNK1-Δ11 and WNK4 constructs (C) or from Xenopus oocytes injected with myc-tagged L-WNK1-Δ11 alone or in combination with WNK4 (D). WNK4 overexpression significantly decreased abundance of L-WNK1-Δ11 in both systems. *: P<0.05 and **: P<0.01 for the comparison between L-WNK1-Δ11 alone and L-WNK1-Δ11 + WNK4 (Student’s unpaired t-test). (E) NCC activity alone or in combination of wild type or mutant-HQ WNKs as stated. The inhibition of the formation of WNKs dimers by mutating the C-terminal HQ motif relieved L-WNK1-Δ11 or WNK3 from WNK4-mediated inhibition. For oocytes experiments, the activity measured in oocytes injected with NCC alone was arbitrarily set to 100% and data are mean ± s.e.m. **: P<0.01 for the comparison between wild type WNK4 and WNK4-HQ (Student’s unpaired t-test).
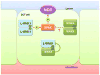
Figure 4. Proposed new model for the regulation of NCC by WNKs
Note that WNK3 is not shown and that L-WNK1 is indicated instead of L-WNK1-Δ11 to simplify the Figure. At baseline, L-WNK1-Δ11 forms homomers that activate NCC in a SPAK-dependent manner. WNK4 could antagonize the effect of L-WNK11-Δ11 on NCC by either forming inactive WNK4-WNK1 heteromers or inhibitory WNK4 homomers.
Similar articles
- Kidney-specific WNK1 isoform (KS-WNK1) is a potent activator of WNK4 and NCC.
Argaiz ER, Chavez-Canales M, Ostrosky-Frid M, Rodríguez-Gama A, Vázquez N, Gonzalez-Rodriguez X, Garcia-Valdes J, Hadchouel J, Ellison D, Gamba G. Argaiz ER, et al. Am J Physiol Renal Physiol. 2018 Sep 1;315(3):F734-F745. doi: 10.1152/ajprenal.00145.2018. Epub 2018 May 30. Am J Physiol Renal Physiol. 2018. PMID: 29846116 Free PMC article. - The thiazide-sensitive Na-Cl cotransporter is regulated by a WNK kinase signaling complex.
Yang CL, Zhu X, Ellison DH. Yang CL, et al. J Clin Invest. 2007 Nov;117(11):3403-11. doi: 10.1172/JCI32033. J Clin Invest. 2007. PMID: 17975670 Free PMC article. - Consequences of SPAK inactivation on Hyperkalemic Hypertension caused by WNK1 mutations: evidence for differential roles of WNK1 and WNK4.
Rafael C, Soukaseum C, Baudrie V, Frère P, Hadchouel J. Rafael C, et al. Sci Rep. 2018 Feb 19;8(1):3249. doi: 10.1038/s41598-018-21405-x. Sci Rep. 2018. PMID: 29459793 Free PMC article. - The interplay of renal potassium and sodium handling in blood pressure regulation: critical role of the WNK-SPAK-NCC pathway.
Wu A, Wolley M, Stowasser M. Wu A, et al. J Hum Hypertens. 2019 Jul;33(7):508-523. doi: 10.1038/s41371-019-0170-6. Epub 2019 Feb 5. J Hum Hypertens. 2019. PMID: 30723251 Review. - Molecular physiology of the thiazide-sensitive sodium-chloride cotransporter.
Ko B, Hoover RS. Ko B, et al. Curr Opin Nephrol Hypertens. 2009 Sep;18(5):421-7. doi: 10.1097/MNH.0b013e32832f2fcb. Curr Opin Nephrol Hypertens. 2009. PMID: 19636250 Free PMC article. Review.
Cited by
- Dietary potassium and the renal control of salt balance and blood pressure.
Penton D, Czogalla J, Loffing J. Penton D, et al. Pflugers Arch. 2015 Mar;467(3):513-30. doi: 10.1007/s00424-014-1673-1. Epub 2015 Jan 6. Pflugers Arch. 2015. PMID: 25559844 Review. - Arterial Blood Pressure, Neuronal Excitability, Mineral Metabolism and Cell Volume Regulation Mechanisms Revealed by Xenopus laevis oocytes.
Gamba G. Gamba G. Membranes (Basel). 2022 Sep 21;12(10):911. doi: 10.3390/membranes12100911. Membranes (Basel). 2022. PMID: 36295670 Free PMC article. - Renal sodium handling and sodium sensitivity.
Frame AA, Wainford RD. Frame AA, et al. Kidney Res Clin Pract. 2017 Jun;36(2):117-131. doi: 10.23876/j.krcp.2017.36.2.117. Epub 2017 Jun 30. Kidney Res Clin Pract. 2017. PMID: 28680820 Free PMC article. Review. - Intracellular chloride: a regulator of transepithelial transport in the distal nephron.
Rodan AR. Rodan AR. Curr Opin Nephrol Hypertens. 2019 Jul;28(4):360-367. doi: 10.1097/MNH.0000000000000502. Curr Opin Nephrol Hypertens. 2019. PMID: 30865168 Free PMC article. Review. - Deletion of KS-WNK1 promotes NCC activation by increasing WNK1/4 abundance.
Ferdaus MZ, Terker AS, Koumangoye RB, Al-Qusairi L, Welling PA, Delpire E. Ferdaus MZ, et al. Am J Physiol Renal Physiol. 2024 Sep 1;327(3):F373-F385. doi: 10.1152/ajprenal.00101.2024. Epub 2024 Jul 4. Am J Physiol Renal Physiol. 2024. PMID: 38961847
References
- Narayan KM, Ali MK, Koplan JP. Global noncommunicable diseases–where worlds meet. N Engl J Med. 2010;363:1196–1198. - PubMed
- Gordon RD, Klemm SA, Tunny TJ, et al. Gordon’s syndrome: A sodium-volume-dependent form of hypertension with a genetic basis. In: Brenner JHLaBM., editor. Hypertension: Patholgy, diagnosis and management. New York: Raven Press Ltd; 1995. pp. 2111–2113.
- Wilson FH, Disse-Nicodeme S, Choate KA, Ishikawa K, Nelson-Williams C, Desitter I, Gunel M, Milford DV, Lipkin GW, Achard JM, Feely MP, Dussol B, Berland Y, Unwin RJ, Mayan H, Simon DB, Farfel Z, Jeunemaitre X, Lifton RP. Human hypertension caused by mutations in wnk kinases. Science. 2001;293:1107–1112. - PubMed
- Uchida S. Regulation of blood pressure and renal electrolyte balance by cullin-ring ligases. Curr Opin Nephrol Hypertens. 2014 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 091415/WT_/Wellcome Trust/United Kingdom
- DK51496/DK/NIDDK NIH HHS/United States
- I01 BX002228/BX/BLRD VA/United States
- Wellcome Trust/United Kingdom
- R01 DK051496/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases