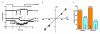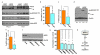Piezo1 integration of vascular architecture with physiological force - PubMed (original) (raw)
. 2014 Nov 13;515(7526):279-282.
doi: 10.1038/nature13701. Epub 2014 Aug 10.
Bing Hou # 1, Sarka Tumova 1, Katsuhiko Muraki 2, Alexander Bruns 1, Melanie J Ludlow 1, Alicia Sedo 1, Adam J Hyman 1, Lynn McKeown 1, Richard S Young 1 3, Nadira Y Yuldasheva 1, Yasser Majeed 1, Lesley A Wilson 1, Baptiste Rode 1, Marc A Bailey 1, Hyejeong R Kim 4, Zhaojun Fu 1, Deborah Al Carter 1, Jan Bilton 1, Helen Imrie 1, Paul Ajuh 5, T Neil Dear 1, Richard M Cubbon 1, Mark T Kearney 1, Raj K Prasad 3, Paul C Evans 4, Justin Fx Ainscough 1, David J Beech 1
Affiliations
- PMID: 25119035
- PMCID: PMC4230887
- DOI: 10.1038/nature13701
Piezo1 integration of vascular architecture with physiological force
Jing Li et al. Nature. 2014.
Abstract
The mechanisms by which physical forces regulate endothelial cells to determine the complexities of vascular structure and function are enigmatic. Studies of sensory neurons have suggested Piezo proteins as subunits of Ca(2+)-permeable non-selective cationic channels for detection of noxious mechanical impact. Here we show Piezo1 (Fam38a) channels as sensors of frictional force (shear stress) and determinants of vascular structure in both development and adult physiology. Global or endothelial-specific disruption of mouse Piezo1 profoundly disturbed the developing vasculature and was embryonic lethal within days of the heart beating. Haploinsufficiency was not lethal but endothelial abnormality was detected in mature vessels. The importance of Piezo1 channels as sensors of blood flow was shown by Piezo1 dependence of shear-stress-evoked ionic current and calcium influx in endothelial cells and the ability of exogenous Piezo1 to confer sensitivity to shear stress on otherwise resistant cells. Downstream of this calcium influx there was protease activation and spatial reorganization of endothelial cells to the polarity of the applied force. The data suggest that Piezo1 channels function as pivotal integrators in vascular biology.
Figures
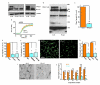
Extended Data Figure 1. Piezo1 mRNA in aorta and endothelial cells
a, End-point PCR products obtained with Piezo1 primers for human (h.) liver and mouse (m.) lung endothelial cell (EC) and freshly-dissected mouse aorta mRNA after reverse transcriptase reaction (RT) to generate cDNA. b, As for (a) but for: human late outgrowth endothelial progenitor cells (LEPC) and 7 types of human endothelial cell (art., arterial; micr., microvascular; pul., pulmonary; umb., umbilical; car., cardiac; bla., bladder; der., dermal; col., colonic). Results are shown with (+ RT) and without (-RT) reverse transcription. c, Quantitative real-time PCR data for experiments of the type shown in (a) (n=2 each in duplicate). d, Quantitative real-time PCR data for experiments of the type shown in (b) (n=1 each in duplicate).
Extended Data Figure 2. Role of Piezo1 channels in HUVEC migration and tube formation
a, Western blot for HUVEC lysate probed with anti-Piezo1 antibody after transfection with the control siRNA (sc.si.), 2 different single Piezo1 siRNAs (P1.si.1 or P1.si.2), or a pooled set of Piezo1 siRNAs (P1.si.3). The upper band in the upper blot represents Piezo1 with a predicted mass of 286 kDa. The band immediately below is unknown protein labeled non-specifically by anti-Piezo1 antibody (*). The lower blot shows β-actin included as a protein-loading control. b, Another western blot for HUVEC lysate probed with anti-Piezo1 antibody after transfection with control siRNA (sc.si.) or Piezo1 siRNA (P1.si.1). The arrow points to Piezo1 protein. Apparent depletion of an additional protein by P1.si.1 is evident at about 130 kDa but this effect was not reproducible in other experiments. Other proteins (e.g. at about 250 (*), 190, and 100 kDa) were non-specifically labeled by the anti-Piezo1 antibody and not affected by Piezo1 siRNA. More specific anti-Piezo1 antibody could not be found. c, Normalized quantitative densitometry analysis for Piezo1 band of the type shown in (b) (n=3). d, Specificity of depletion by Piezo1 siRNA. The effect of the TRPV4 channel activator 10 μM 4α-phorbol 12,13-didecanoate (4-αPDD) is shown on intracellular Ca2+ in HUVECs in multiple wells of a 96-well plate on a fluorescence plate-reader (representative of n=3). HUVECs were transfected with sc.si. or P1.si.1. The data show that P1.si.1 did not affect TRPV4. e, Cell migration after transfection with sc.si compared with P1.si.1 or sc.si. compared with P1.si.2 (n=4 each). f, As for (e) but comparing vehicle controls with 5 μM GsMTx4 or 30 μM ruthenium red (n=3 each). g, Example images of in vitro tube formations in co-culture with fibroblasts. HUVECs were transfected with sc.si or P1.si.1 and labeled with anti-CD31 antibody (green). Scale bar, 400 μm. h, Analysis of tube length in images of the type shown in (g) and similarly for P1.si.2 (n=3 for all groups). i, Example sections from in vivo Matrigel plugs in which HUVECs were transfected with sc.si. or P1.si.1. The arrow points to a typical tube structure. Scale bar, 50 μm. j, Mean data from tubes exemplified by (i) for 6 independent experiments (i-vi) (5-17 tissue sections each). Error bars are s.e.m.
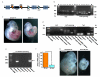
Extended Data Figure 3. Global and endothelial-specific Piezo1 modification and embryonic growth retardation in mice
a, Simplified diagram of the Piezo1 Knockout First (conditional) construct provided in ES cells by the KOMP Repository (
). Piezo1 is indicated containing insertion of lacZ sequence flanked by flippase recognition target (FRT) sites and downstream loxP sites. Further details of the construct can be obtained at
. b-c, Global modification. b, Example genotyping results with lacZ or loxP-spanning PCR primers. M indicates the DNA marker ladder. On the left are results for 6 mice analysed by the lacZ PCR primers (expected product: 225 bp). On the right are the results for the same 6 mice analysed by primers targeted to endogenous Piezo1 sequence either side of the 3′ terminal loxP site (expected products: 155 bp without the loxP site; 189 bp with the loxP site). In the gel shown, 3 mice were heterozygous for the construct (+/−), 2 homozygous (−/−), and 1 wild-type (+/+). c, Images of example sibling E10.5 embryos. The embryo on the left was Piezo1+/+ and the embryo on the right was _Piezo1_−/−. The scale bar is 1 mm. d-g, Endothelial-specific modification. d, Example genotyping results for two mice (mouse 1 and mouse 2) both with deletion of the lacZ insert and transmission of Tie2-cre. Controls for the absence and presence of lacZ, the loxP insert, and Tie2-cre are included. Successful deletion of the lacZ insert was confirmed by lack of β-galactosidase staining (data not shown). e, Example genotyping results for six sibling embryos analysed with PCR primers spanning the deletion predicted to result from cre recombinase activity at the loxP sites. The forward primer was 5′ of the 5′ FRT site illustrated in (a) and the reverse primer was 3′ of the 3′ loxP site. The PCR product size after deletion was expected to be 379 bp. The product was detected in embryos 2 and 6. The PCR product was not generated in embryos without the deletion because it was too long to be amplified (4208 bp). Embryos exhibiting the 379 bp product were designated “EC-del.” to indicate disruptive deletion in Piezo1 of endothelial cells (ECs). Embryos designated as wild-type (wt.) exhibited no 379 bp product and only the 155 bp loxP product (as shown for the “no loxP insert control” in d). Out of a total of 142 embryos, 57 were EC-del. f, RT-PCR products detecting Piezo1 mRNA in total RNA from sibling embryos (Piezo1 3′ PCR primers) (n=3, each in duplicate). Piezo1 mRNA was significantly depleted in embryos displaying the 379 bp product described and shown in (e). g, Images of example sibling E10.5 embryos. The embryo on the left was wild-type and the embryo on the right contained endothelial-specific Piezo1 deletion (EC-del.). Retarded growth was apparent in EC-del. embryos and none of the other embryos. The scale bar is 1 mm. Error bars are s.e.m.
Extended Data Figure 4. Piezo1-dependence of shear stress-evoked Ca2+ events in human endothelial cells and mouse embryonic endothelial cells
a, Example intracellular Ca2+ events evoked by microfluidic shear stress in HUVECs transfected with control siRNA (sc.si.) or Piezo1.si.1 (P1.si.1). Each trace is for 1 cell. In one P1.si.1 cell, transient Ca2+ elevation remained. Such residual events may reflect insufficient Piezo1 depletion in some cells or non-Piezo1 mechanisms. b, Mean data for experiments of the type in (a) and expanded to paired comparisons of sc.si. and P1.si.1 (n=5 each), sc.si. and P1.si.2 (n=4 each), vehicle and 2.5 μM GsMTx4 (n=3 each). Data were normalized to their respective controls. c, Quantification of Piezo1 mRNA depletion (n=4 each) plotted against the inhibition of the intracellular Ca2+ elevations evoked by 20 dyn.cm−2. Three different Piezo1 siRNAs were compared with their control siRNAs. The Ca2+ data are from the experiments described in (b). Sequence details of the siRNAs are provided in Table S3. d, Mean Ca2+ signals evoked by 20 dyn.cm−2 in non-transfected HUVECs. Measurements were made in standard bath solution without the addition of an inhibitor (no inhibitor) (n=8), 10 μM gadolinium chloride (Gd3+) (n=3), or with Ca2+ omitted from the bath solution (0 Ca2+) (n=3). e, Ca2+ release evoked by 2 μM thapsigargin (TG) in the absence of extracellular Ca2+ and after transfection with sc.si. or P1.si.1 (20 wells of a 96-well plate each). f, Mean data normalized to sc.si. for experiments of the type shown in (e) and analysed for the rate of rise of the Ca2+ event evoked by TG (n=3 each). g, Similar to (b) but endothelial cells were from patient liver samples, data were not normalized, and only P1.si.1 was used (n=3, 4, 10 and 5 for shear stresses of 5, 10, 15 and 20 dyn.cm−2). h, i, Intracellular Ca2+ measurements from mouse embryonic endothelial cells in microfluidic chambers. h, Superimposition of example intracellular Ca2+ events in 2 single cells on different coverslips from Piezo1+/+ and _Piezo1_−/− sibling embryos. Shear stress was applied at 15 and 25 dyn.cm−2 and then 30 ng.mL−1 VEGF was introduced while maintaining shear stress at 25 dyn.cm−2. i, Mean±s.e.mean data for all VEGF-responsive cells studied as exemplified in (h) (n=6 +/+, 54 cells; n=5 −/−, 42 cells). The same data are summarized in simplified form in Fig 2a. Error bars are s.e.m.
Extended Data Figure 5. Piezo1-dependence of mechanically-activated single channels in HUVECs
a, Example single channel currents in a cell-attached patch at three voltages without subtraction of holding current. Application of −15 mmHg pressure steps to the patch pipette evoked open channel unitary currents that summated to two levels marked as O1 and O2. Closed channel current is indicated by C. b, Mean amplitudes of unitary events as exemplified in (a) and fitted with a straight line (3 patches for −50, −30 and −50 mV; 1 patch for +30 mV). c, Paired comparisons of the percentage of patches containing channel events exemplified in (a) for cells transfected with sc.si. or P1.si.1 in two independent experiment groups (n values for each group are in parentheses). In Group 2 cell-attached patch recordings cells were exposed for 10 min to 0.4 mM EGTA to chelate contaminating Ca2+ prior to recording so that sc.si.- and P1.si.1-treated cells rounded up similarly; without this treatment (Group 1), P1.si.1 but not sc.si. cells tended to round up in response to the high-K+ bath solution used to null the membrane potential of cells in cell-attached patch recordings (the reason for this effect is unknown but it may relate to changes in cytoskeleton and adhesion as discussed in relation to Fig 4). Error bars are s.e.m.
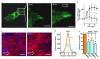
Extended Data Figure 6. Shear stress-evoked redistribution of Piezo1 and the role of Piezo1 in alignment of endothelial cells to the direction of shear stress
The application and direction of shear stress is indicated by open arrows and the cells were HUVECs. a, The left-hand image is of Piezo1-GFP in a single cell with a box indicating the region expanded in the middle and right-hand images after 0 and 50 min 15 dyn.cm−2 in the microfluidic chamber. In the left image i indicates the part of the cell that became trailing after application of shear stress and ii that which became leading. Scale bars, 10 μm. b, Analysis of experiments of the type shown in (a) (n=8 per data point except for n=7 at 50 min) where i and ii indicate the trailing and leading edges of the cell as shown in (a). c, Example cells after 24 h shear stress caused by the orbital shaker. Rhodamine phalloidin labeled F-actin (red) and DAPI labeled cell nuclei (blue). A paired comparison was made of cells transfected with control siRNA (sc.si.) or Piezo1 siRNA (P1.si.1). Scale bars, 50 μm. d, Example orientation analysis for pairs of images of the type shown in (c). e, As for (d) but normalized mean data for the frequency (number of angles) at the mode in experiments comparing mock with P1.si.1 transfected cells (n=5 each) and 2.5 μM GsMTx4 with its vehicle control (n=4 each). There is also comparison of cells transfected with sc.si. or P1.si.1 after 15 h of 15 dyn.cm−2 in the microfluidic chamber (n=3). Error bars are s.e.m.
Extended Data Figure 7. Coupling to endothelial nitric oxide synthase
a, Western blot for HUVEC lysates probed with anti-Piezo1 antibody after transfection with Piezo1 siRNA P1.si.1 (on the left) or the control siRNA sc.si. (on the right). Prior to collection of cell lysates, HUVECs were treated with 30 ng.mL−1 VEGF (+) or no VEGF (-) for 10 min. The lysate was probed with anti-Piezo1 antibody, antibody to phosphorylated S1177 in eNOS, anti-β-actin antibody, and antibody to total eNOS protein. Positions of the expected proteins are indicated by the text on the right. The non-specific band at 250 kDa in the anti-Piezo1 blot is highlighted with *, as in Extended Data Figure 2a, b. b, Quantitative data for the down-regulation of total eNOS after transfection of HUVECs with P1.si.1 (n=6). c, Fold-change in S1177 eNOS phosphorylation (p-eNOS) evoked by VEGF (30 ng.mL−1) in HUVECs transfected with control siRNA (sc.si). or Piezo1 siRNA (P1.si.1) (n=3 each). The grey dashed line highlights 1-fold (i.e. no change). d, Western blot for VEGF (30 ng.mL−1) evoked S1177 eNOS phosphorylation (arrow) in aorta. Aorta was dissected from Piezo1+/+ or Piezo1+/− litter-mates and allowed to equilibrate at 37 oC in culture medium without shear stress for 3 h. Aorta was then exposed to VEGF (30 ng.mL−1) (+VEGF) or not (-VEGF) for 10 min, after which lysates were generated. Proteins were probed with antibody to phosphorylation at S1177 in eNOS. The band labeled with ** was not included in the analysis. The blot was also probed with anti-β-actin antibody to test for equal protein loading. e, Mean data for the type of experiment exemplified in (d) (n=5 for each genotype) and presented as in (c). f, Western blotting for HUVEC lysates after transfection with control siRNA (sc.si.) or eNOS siRNAs. The blot was probed with anti-eNOS (total) antibody. g, HUVEC migration to VEGF after incubation with vehicle control, 0.3 mM L-NMMA for 0.5 h, or 48 h after transfection with sc.si. or one of three siRNAs targeted to eNOS (n=3 each; each paired to its own control). h, Data interpretation. Error bars are s.e.m.
Extended Data Figure 8. Endothelial cell alignment to shear stress lacks dependency on nitric oxide but is coupled to calpain
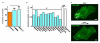
a, Frequency of HUVEC alignment induced on the orbital shaker. Data for each test condition were normalized to their own control. Test conditions were 0.3 mM L-NMMA (n=3) and transfection with eNOS siRNA (eNOS.si.1) compared with mock transfection (n=4). b, Protein abundances from mass spectrometry analysis for the indicated proteins in 3 _Piezo1_−/− relative to 3 Piezo1+/+ E10.5 embryos. Calpain-2 and its substrates were less in _Piezo1_−/− embryos. The effects were relatively specific because more than 1300 of the detected proteins were unchanged by Piezo1 depletion; data for 2 examples (myosin light chain-3 and integrin-β1) are shown. c, Fluorescence images of Piezo1-GFP in a HUVEC before (upper image) and after (lower image) 15 dyn.cm−2 for 50 min. The small solid arrows point to focal adhesion structures at the trailing edge of the cell. Scale bar, 10 μm. Representative of n=4. Error bars are s.e.m.
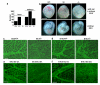
Figure 1. Piezo1 in murine embryos
a, Numbers born or detected as embryos with Piezo1+/+, Piezo1+/− or _Piezo1_−/− genotypes. b, Images of sibling yolk sacs (containing embryos) and embryos at E9.5. Scale bar, 1 mm. (c, d) Dissected Piezo1+/+ and _Piezo1_−/−yolk sacs stained for CD31. (c) E9.5. Typical of n=11 (+/+) and n=8 (−/−). d, E10.5. Typical of n=9 (+/+) and n=11 (−/−). e, f, As for (c, d) except wild-type (EC-wt.) and endothelial-specific _Piezo1_-modified (EC-del.) embryos. e, E9.5. Typical of n=6 (EC-wt.) and n=9 (EC-del.). f, E10.5. Typical of n=5 (EC-wt.) and n=11 (EC-del.). Scale bars, 100 μm.
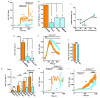
Figure 2. Piezo1 in shear stress sensing
a, Ca2+ elevation evoked in endothelial cells from Piezo1+/+ (n=6) and _Piezo1_−/− (n=5) E9.5 embryos. b, Whole-cell current in a HUVEC exposed to 12, 5, 8, and 12 μL.s−1 super-fusion (flow). c, I-Vs for i and ii as indicated in (b). d, Paired comparison of 12 μL.s−1 responses at −24 mV as in (b): control siRNA (sc.si.) n=6, P1.si.1 n=7. e, Ca2+ in HEK 293 cells without transfection (non-trans., 5 cells) or transfected with wild-type (WT) Piezo1-GFP (7 cells) (n=1). f, Mean data of the type in (e) and for M2225R mutant (n=6 each). Error bars are s.e.m.
Figure 3. Piezo1 in endothelial cell alignment
a, Endothelial cells from E9.5 Piezo1+/+ and _Piezo1_−/− embryos with no shear stress (left) or 15 dyn.cm−2 as indicated by arrows. CD31 (green, left only), F-actin (red), nuclei (blue). Scale bars, 50 μm. b, Mean data for experiments as in (a) (n=4 +/+, n=5 −/−). c, Cerebral artery Piezo1 mRNA abundance detected 5′ and 3′ of _Piezo1_-disruption (n=2 each for Piezo1+/+ and Piezo1+/−). d, Cerebral arteries labeled for CD31 (green) and nuclei (blue). Scale bar, 40 μm. e, Quantification of in vivo CD31 orientation as shown in (d) (n=4 each). Error bars are s.e.m.
Figure 4. Piezo1 coupling to calpain
a, Calpain activity indicated as absorbance (abs.) in embryos at E9.5 (n=3 Piezo1+/+, n=3 _Piezo1_−/−) and E10.5 (n=3 Piezo1+/+, n=3 _Piezo1_−/−). b, Calpain activity in HUVECs without or with shear stress (orbital shaker) for 15 min (n=3). GsMTx4 (2.5 μM). c, HUVEC alignment analysis as in Extended Data Fig. 6d, e. Test conditions: nominally Ca2+-free Krebs solution (0 Ca2+) (n=3); 3 μM PD150606 (calpain inhibitor) (n=3); 20 μM PD151746 (calpain inhibitor) (n=3); 20 μM PD145305 (negative control) (n=3); 25 μM CK59 (CaMKII inhibitor) (n=3); and 6 μM CN585 (calcineurin inhibitor) (n=3). d, Data interpretation. Error bars are s.e.m.
Comment in
- Endothelial Piezo1: life depends on it.
Li J, Hou B, Beech DJ. Li J, et al. Channels (Austin). 2015;9(1):1-2. doi: 10.4161/19336950.2014.986623. Channels (Austin). 2015. PMID: 25558961 Free PMC article. No abstract available.
Similar articles
- Piezo1 Channels in Vascular Development and the Sensing of Shear Stress.
Hyman AJ, Tumova S, Beech DJ. Hyman AJ, et al. Curr Top Membr. 2017;79:37-57. doi: 10.1016/bs.ctm.2016.11.001. Epub 2017 Jan 10. Curr Top Membr. 2017. PMID: 28728823 Review. - Endothelial Piezo1: life depends on it.
Li J, Hou B, Beech DJ. Li J, et al. Channels (Austin). 2015;9(1):1-2. doi: 10.4161/19336950.2014.986623. Channels (Austin). 2015. PMID: 25558961 Free PMC article. No abstract available. - Piezo1 channels are mechanosensors in human fetoplacental endothelial cells.
Morley LC, Shi J, Gaunt HJ, Hyman AJ, Webster PJ, Williams C, Forbes K, Walker JJ, Simpson NAB, Beech DJ. Morley LC, et al. Mol Hum Reprod. 2018 Oct 1;24(10):510-520. doi: 10.1093/molehr/gay033. Mol Hum Reprod. 2018. PMID: 30085186 Free PMC article. - Piezo1, a mechanically activated ion channel, is required for vascular development in mice.
Ranade SS, Qiu Z, Woo SH, Hur SS, Murthy SE, Cahalan SM, Xu J, Mathur J, Bandell M, Coste B, Li YS, Chien S, Patapoutian A. Ranade SS, et al. Proc Natl Acad Sci U S A. 2014 Jul 15;111(28):10347-52. doi: 10.1073/pnas.1409233111. Epub 2014 Jun 23. Proc Natl Acad Sci U S A. 2014. PMID: 24958852 Free PMC article. - Endothelial Piezo1 channels as sensors of exercise.
Beech DJ. Beech DJ. J Physiol. 2018 Mar 15;596(6):979-984. doi: 10.1113/JP274396. Epub 2018 Jan 9. J Physiol. 2018. PMID: 29194632 Free PMC article. Review.
Cited by
- Confinement Sensing and Signal Optimization via Piezo1/PKA and Myosin II Pathways.
Hung WC, Yang JR, Yankaskas CL, Wong BS, Wu PH, Pardo-Pastor C, Serra SA, Chiang MJ, Gu Z, Wirtz D, Valverde MA, Yang JT, Zhang J, Konstantopoulos K. Hung WC, et al. Cell Rep. 2016 May 17;15(7):1430-1441. doi: 10.1016/j.celrep.2016.04.035. Epub 2016 May 5. Cell Rep. 2016. PMID: 27160899 Free PMC article. - Ionic Selectivity and Permeation Properties of Human PIEZO1 Channels.
Gnanasambandam R, Bae C, Gottlieb PA, Sachs F. Gnanasambandam R, et al. PLoS One. 2015 May 8;10(5):e0125503. doi: 10.1371/journal.pone.0125503. eCollection 2015. PLoS One. 2015. PMID: 25955826 Free PMC article. - Modeling and live imaging of mechanical instabilities in the zebrafish aorta during hematopoiesis.
Chalin D, Bureau C, Parmeggiani A, Rochal S, Kissa K, Golushko I. Chalin D, et al. Sci Rep. 2021 Apr 29;11(1):9316. doi: 10.1038/s41598-021-88667-w. Sci Rep. 2021. PMID: 33927284 Free PMC article. - Identifying the causes of recurrent pregnancy loss in consanguineous couples using whole exome sequencing on the products of miscarriage with no chromosomal abnormalities.
Najafi K, Mehrjoo Z, Ardalani F, Ghaderi-Sohi S, Kariminejad A, Kariminejad R, Najmabadi H. Najafi K, et al. Sci Rep. 2021 Mar 26;11(1):6952. doi: 10.1038/s41598-021-86309-9. Sci Rep. 2021. PMID: 33772059 Free PMC article. - Mechanical Intercellular Communication via Matrix-Borne Cell Force Transmission During Vascular Network Formation.
Davidson CD, Midekssa FS, DePalma SJ, Kamen JL, Wang WY, Jayco DKP, Wieger ME, Baker BM. Davidson CD, et al. Adv Sci (Weinh). 2024 Jan;11(3):e2306210. doi: 10.1002/advs.202306210. Epub 2023 Nov 23. Adv Sci (Weinh). 2024. PMID: 37997199 Free PMC article.
References
- Ando J, Yamamoto K. Flow detection and calcium signaling in vascular endothelial cells. Cardiovasc Res. 2013;99:260–268. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- G0901761(93442)/MRC_/Medical Research Council/United Kingdom
- G1002076/MRC_/Medical Research Council/United Kingdom
- RG/13/1/30042/BHF_/British Heart Foundation/United Kingdom
- 083857/WT_/Wellcome Trust/United Kingdom
- BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- FS/14/22/30734/BHF_/British Heart Foundation/United Kingdom
- WT_/Wellcome Trust/United Kingdom
- FS/12/54/29671/BHF_/British Heart Foundation/United Kingdom
- MR/L019051/1/MRC_/Medical Research Council/United Kingdom
- RG/09/010/28087/BHF_/British Heart Foundation/United Kingdom
- FS/12/80/29821/BHF_/British Heart Foundation/United Kingdom
- CRUK_/Cancer Research UK/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous