Matrix elasticity regulates lamin-A,C phosphorylation and turnover with feedback to actomyosin - PubMed (original) (raw)
Matrix elasticity regulates lamin-A,C phosphorylation and turnover with feedback to actomyosin
Amnon Buxboim et al. Curr Biol. 2014.
Abstract
Tissue microenvironments are characterized not only in terms of chemical composition but also by collective properties such as stiffness, which influences the contractility of a cell, its adherent morphology, and even differentiation. The nucleoskeletal protein lamin-A,C increases with matrix stiffness, confers nuclear mechanical properties, and influences differentiation of mesenchymal stem cells (MSCs), whereas B-type lamins remain relatively constant. Here we show in single-cell analyses that matrix stiffness couples to myosin-II activity to promote lamin-A,C dephosphorylation at Ser22, which regulates turnover, lamina physical properties, and actomyosin expression. Lamin-A,C phosphorylation is low in interphase versus dividing cells, and its levels rise with states of nuclear rounding in which myosin-II generates little to no tension. Phosphorylated lamin-A,C localizes to nucleoplasm, and phosphorylation is enriched on lamin-A,C fragments and is suppressed by a cyclin-dependent kinase (CDK) inhibitor. Lamin-A,C knockdown in primary MSCs suppresses transcripts predominantly among actomyosin genes, especially in the serum response factor (SRF) pathway. Levels of myosin-IIA thus parallel levels of lamin-A,C, with phosphosite mutants revealing a key role for phosphoregulation. In modeling the system as a parsimonious gene circuit, we show that tension-dependent stabilization of lamin-A,C and myosin-IIA can suitably couple nuclear and cell morphology downstream of matrix mechanics.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Figures
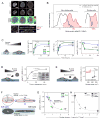
Figure 1. Increased stress on the nucleus suppresses lamin-A,C phosphorylation
(A) Lamin-A,C pSer22 is present in interphase cells. Top two rows: confocal image stacks of total and pSer22 lamin-A,C in MSCs fixed after 1-to-24 hours of adhesion showed wrinkled nuclei at early stages of cell adhesion that stretched and smoothed with spreading. Third row: pSer22/LMNA ratios from the top two panels, calculated pixel-by-pixel and normalized to mean fold-change. After 24 hrs, greater phospho-LMNA concentration was observed in the nucleoplasm vs. at the nuclear periphery. Bottom row: Confocal cross-sections confirmed nucleoplasmic pSer22 (all scale bars = 10 μm). (B) Histogram of pSer22 levels in a population of A549 cells (Figs. S1A–D), showing specificity of pSer22 immunofluorescence and stoichiometry calibrated by MS (Fig. S1E). Dividing cells showed the greatest extent of phosphorylation (Fig. S1C). Pre-incubation with a phospho-epitope blocking peptide decreased immunofluorescence intensity, as did non-specific binding to a non-phosphorylated version of the same peptide, but to a significantly lesser extent (Fig. S1D). In the absence of primary antibody (2° antibody only), fluorescence intensity was very low (n = 33–249 cells per group). (C) Stiff matrix enhances nuclear stress which inhibits phosphorylation of lamin-A,C. Stress on nuclei in MSCs was manipulated by changing matrix stiffness and adhesion time. Cells spread, with correspondingly greater projected nuclear areas, with increasing adhesion time, and to a greater extent on stiff (40 kPa, black line) vs. soft (0.3 kPa, blue line) substrate. A thin, soft matrix (0.3 kPa, green line) showed behavior intermediate between soft and stiff. The matrix-modulated adhesion process was accompanied by an increase in the total LMNA and a reduction in the fraction pSer22/LMNA. Error bars in all plots show ± SEM (n = 77–276 cells per group). (D) Cell detachment increased pSer22/LMNA in rounding nuclei, with phosphorylation (quantified by immunoblot; n = 3) increasing over tens of minutes in suspension, consistent with relaxation of nuclear stress. (E) pSer22/LMNA increased with myosin-IIA inhibition and stress relaxation by blebbistatin (blebb) treatment (immunofluorescence, Fig. S1E) (n = 25–32 cells per group). (F) Nuclear shape was modeled as an ellipsoid, with surface area assumed to be constant during nuclear deformation. During cell spreading, the nucleus was stretched and flattened down against the substrate, subject to tangential stretching (‘hoop strain’) but negligible radial strain. As the hoop strain scaled with the radius r, and the fold-change in r can be estimated by the square root of the fold-change in projected nuclear area, we concluded that the nuclear strain involved in cell spreading can be estimated by ε~a/a0, where a is the nuclear projected area and _a_0 corresponds to the initial state. (G) By expressing nuclear tension as a function of lamin-A,C level and nuclear area, pSer22/LMNA data was fit to hyperbolic decays. Plot shows data fitting for MSCs cultured on soft, soft/thin and stiff hydrogel substrates (adhesion time: 1, 3.5 and 24 hrs; Fig. 1C). (H) Data fitting for MSCs subjected to lamin-A,C KD and blebbistatin treatment (Figs. 1E; S1E).
Figure 2. Phosphomimetic lamin-A constructs show increased mobility and soften the nucleus
(A) The mobile fraction, f, of GFP-fused wild-type lamin-A and phosphomutant lamin-A constructs (S22D and S22A) was evaluated by fitting an exponential curve to the recovering intensities of wide side-to-side bands photobleached in the nuclei of MSCs. (B) Analysis of FRAP experiments showed that the WT construct was comparatively more mobile in MSCs cultured for 2 hrs on soft (0.3 kPa) than on stiff (40 kPa) substrate (Fig. S2A). Independent of matrix stiffness, S22D mobile fractions were higher than WT, whereas S22A remained polymerized (Fig. S2B). WT lamin-A was solubilized by blebb after 24 hours on stiff gels, but treatment of phosphomimetic S22D showed no significant change (n = 9–18 nuclei per group). (C) Live-cell imaging of WT, S22D and S22A phospho-mimetic GFP-lamin-A constructs. S22D showed a diffusive nucleoplasmic distribution and low nuclear spreading, consistent with a nucleoplasmic distribution of pSer22. (D) S22D and S22A suppressed matrix mechanosensitivity of MSCs whereas WT nuclei spread by > 2-fold on stiff matrix (40 kPa) relative to soft matrix (0.3 kPa) (n = 5–18 nuclei per group). (E) Relaxation times measured by micropipette aspiration showed S22D nuclei to be significantly softer than WT nuclei, and S22A nuclei were moderately stiffer. Relaxation times were determined at the same value of creep compliance (J = 1.4 kPa−1) per [21]. Error bars show log mean ± SEM (n > 4 nuclei).
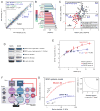
Figure 3. Phosphorylation of lamin-A,C promotes proteolysis and nuclear softening
(A) Phospho-lamin-A,C is present in both full-length and cleaved states. MSCs lysed following short (10 min) and long (45 min) periods of rounding in suspension showed numerous low-MW, fragmentation-product bands in immunoblots against lamin-A,C and, to a greater extent, against pSer22. (B) Profiles of immunoblots, showing MW ranges analyzed by MS (densitometry shown in Figs. S3A, B). (C) Gel slices A-to-D were analyzed by MS, confirming the existence of low MW lamin-A cleavage products (peptide coverage of 25 – 40 kDa range shown; blue-to-red coloring indicates peptides detected with increased ion current). Phosphorylation sites detectable by MS are indicated in yellow. (D) All examined bands had lamin-A fragments (see Fig. S3C for sequence coverage maps). (E) Inhibition of lamin-A,C phosphorylation suppresses cleavage. Immunoblots against lamin-A pSer22 or lamin-A cleavage product(s). The latter antibody shows minimal detection of intact lamin-A and a high intensity band at 40 kDa. (F) Treatment with CDK inhibitor RO3306 reduced the extent of phosphorylation at Ser22 in MSCs. (G) RO3306 also suppressed the formation of lamin-A,C cleavage products, as determined by two independent antibodies (see Figs. S3F–H for representative images and analysis). (H) Drug induced lamin-A,C phospho-inhibition stiffened the nucleus as determined by micropipette aspiration (relaxation times determined at constant compliance, J = 1.25 kPa−1 per [21]). Error bars show log mean ± SEM (n > 4 nuclei). (I) MS showed a correlated increase in phosphorylation at S22 and S390 during lamin-A overexpression in A549 cells. (J) Phosphorylation at S22 and S390 showed a correlated decrease during lamin-A,C KD in MSCs (error bars show ± SEM; see Fig. S1D for calibration of phosphorylation measurements by MS).
Figure 4. Lamin-A,C level and phosphorylation state regulate myosin-IIA, factors that can be combined into a mechano-sensitive gene circuit model
(A) Lamin-A,C level regulates myosin-IIA through the Serum Response Factor (SRF) pathway. Transcriptional profiling of MSCs subjected to lamin-A,C KD (n = 3). SRF and cofactors (in cyan) and target genes (blue) were significantly suppressed. The YAP1-regulated Hippo pathway has also been implicated in cellular mechanosensitivity [7], but appeared unaffected here. (B) Transcripts of SRF and cofactors (cyan) were suppressed with lamin-A,C KD, along with those of multiple actin-binding nuclear envelope proteins (red) (n = 3) (see Fig. S4A for GO-term analysis). (C) Correlation between protein and transcript changes with lamin-A,C KD in MSCs. Points represent 485 proteins quantified by MS with three-or-more peptides per protein, and their associated mRNA quantified by DNA microarray. Each point is averaged from three biological replicates and the genes/proteins that are significantly perturbed (p < 0.05) counted in the four quadrants of the plot. Of genes with GO annotation “actin cytoskeleton”, (_n_ = 52, in red), 14 are unchanged and 36 have reduced levels of protein and mRNA. Of genes classified as SRF targets (_n_ = 12) by Olson et al, four are unchanged and eight – including myosin-IIA (_MYH9_) – have reduced levels of protein and mRNA [30]. (**D**) S22A and E GFP-lamin mutants expressed in cells with knockdown of endogenous lamin-A,C show roughly similar levels of both constructs and endogenous protein. (**E**) Lamin-A,C phosphorylation feeds back into myosin-IIA level. Expression of increasing levels of phosphomimetic GFP-S22E-lamin-A in A549 cells with KD of endogenous lamin-A,C had minimal effect on myosin-IIA levels. In contrast, expression of an non-phosphorylatable S22A construct caused a relatively increased quantity of myosin-IIA (x-axis shows total LMNA; S22A data fit by hyperbolic function: _y_ = _abx_ (1 + _bx_)−1 + _c_ (_a_ = 1.3; _b_ = 5.5; _c_ = 4.3; _R_2 > 0.95); each point is averaged data from n > 20 cells; see Figs. S4B–D for representative images and analysis of cell morphology). (F) Tension dependent phosphorylation and turnover feeds into transcriptional regulation, as captured by a Mechanobiological Gene Circuit (MGC) systems model that couples both lamin-A,C and myosin-IIA levels to matrix stiffness. Lamin-A,C transcriptionally regulates LMNA via the retinoic acid pathway (through a mediator, α) and also MYH9 via the SRF pathway through nuclear actin. On stiff matrix, non-phosphorylated, contraction-competent myosins positively regulate lamin-A,C favoring assembly and opposing degradation that occurs on soft matrices. (G) The MGC systems model predicted that lamin-A,C and myosin-IIA levels should monotonically increase with matrix elasticity but also saturate on rigid substrates. (H) MGC model suggests decreased lamin phosphorylation with increasing nuclear tension (consistent with model and experimental data shown in Figs. 1F–H).
Similar articles
- Coordinated increase of nuclear tension and lamin-A with matrix stiffness outcompetes lamin-B receptor that favors soft tissue phenotypes.
Buxboim A, Irianto J, Swift J, Athirasala A, Shin JW, Rehfeldt F, Discher DE. Buxboim A, et al. Mol Biol Cell. 2017 Nov 7;28(23):3333-3348. doi: 10.1091/mbc.E17-06-0393. Epub 2017 Sep 20. Mol Biol Cell. 2017. PMID: 28931598 Free PMC article. - Progerin phosphorylation in interphase is lower and less mechanosensitive than lamin-A,C in iPS-derived mesenchymal stem cells.
Cho S, Abbas A, Irianto J, Ivanovska IL, Xia Y, Tewari M, Discher DE. Cho S, et al. Nucleus. 2018 Jan 1;9(1):230-245. doi: 10.1080/19491034.2018.1460185. Nucleus. 2018. PMID: 29619860 Free PMC article. - Nuclear lamin-A scales with tissue stiffness and enhances matrix-directed differentiation.
Swift J, Ivanovska IL, Buxboim A, Harada T, Dingal PC, Pinter J, Pajerowski JD, Spinler KR, Shin JW, Tewari M, Rehfeldt F, Speicher DW, Discher DE. Swift J, et al. Science. 2013 Aug 30;341(6149):1240104. doi: 10.1126/science.1240104. Science. 2013. PMID: 23990565 Free PMC article. - Nucleoskeletal stiffness regulates stem cell migration and differentiation through lamin A/C.
Chen L, Jiang F, Qiao Y, Li H, Wei Z, Huang T, Lan J, Xia Y, Li J. Chen L, et al. J Cell Physiol. 2018 Jul;233(7):5112-5118. doi: 10.1002/jcp.26336. Epub 2018 Jan 23. J Cell Physiol. 2018. PMID: 29215717 Review. - Crucial Role of Lamin A/C in the Migration and Differentiation of MSCs in Bone.
Alcorta-Sevillano N, Macías I, Rodríguez CI, Infante A. Alcorta-Sevillano N, et al. Cells. 2020 May 26;9(6):1330. doi: 10.3390/cells9061330. Cells. 2020. PMID: 32466483 Free PMC article. Review.
Cited by
- Systems mechanobiology: tension-inhibited protein turnover is sufficient to physically control gene circuits.
Dingal PC, Discher DE. Dingal PC, et al. Biophys J. 2014 Dec 2;107(11):2734-43. doi: 10.1016/j.bpj.2014.10.042. Epub 2014 Dec 2. Biophys J. 2014. PMID: 25468352 Free PMC article. - Towards an understanding of the mechanoreciprocity process in adipocytes and its perturbation with aging.
De Luca M, Mandala M, Rose G. De Luca M, et al. Mech Ageing Dev. 2021 Jul;197:111522. doi: 10.1016/j.mad.2021.111522. Epub 2021 Jun 18. Mech Ageing Dev. 2021. PMID: 34147549 Free PMC article. Review. - Cancer cell migration in 3D tissue: negotiating space by proteolysis and nuclear deformability.
Krause M, Wolf K. Krause M, et al. Cell Adh Migr. 2015;9(5):357-66. doi: 10.1080/19336918.2015.1061173. Epub 2015 Aug 24. Cell Adh Migr. 2015. PMID: 26301444 Free PMC article. - Discovery of surface biomarkers for cell mechanophenotype via an intracellular protein-based enrichment strategy.
Dempsey ME, Chickering GR, González-Cruz RD, Fonseca VC, Darling EM. Dempsey ME, et al. Cell Mol Life Sci. 2022 May 27;79(6):320. doi: 10.1007/s00018-022-04351-w. Cell Mol Life Sci. 2022. PMID: 35622146 Free PMC article. - Targeting Nuclear Mechanics Mitigates the Fibroblast Invasiveness in Pathological Dermal Scars Induced by Matrix Stiffening.
Fu X, Taghizadeh A, Taghizadeh M, Li CJ, Lim NK, Lee JH, Kim HS, Kim HW. Fu X, et al. Adv Sci (Weinh). 2024 Apr;11(15):e2308253. doi: 10.1002/advs.202308253. Epub 2024 Feb 14. Adv Sci (Weinh). 2024. PMID: 38353381 Free PMC article.
References
- Discher DE, Janmey P, Wang YL. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;310:1139–1143. - PubMed
- Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. - PubMed
- Even-Ram S, Doyle AD, Conti MA, Matsumoto K, Adelstein RS, Yamada KM. Myosin IIA regulates cell motility and actomyosin microtubule crosstalk. Nat Cell Biol. 2007;9:299–U104. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R21 EB004000/EB/NIBIB NIH HHS/United States
- R01 HL062352/HL/NHLBI NIH HHS/United States
- 8UL1TR000003/TR/NCATS NIH HHS/United States
- P30DK090969/DK/NIDDK NIH HHS/United States
- R01HL062352/HL/NHLBI NIH HHS/United States
- S10 RR022575/RR/NCRR NIH HHS/United States
- P01 DK032094/DK/NIDDK NIH HHS/United States
- R01 EB007049/EB/NIBIB NIH HHS/United States
- R01EB007049/EB/NIBIB NIH HHS/United States
- R21 AI087516/AI/NIAID NIH HHS/United States
- UL1 TR000003/TR/NCATS NIH HHS/United States
- R21 EB004489/EB/NIBIB NIH HHS/United States
- P01DK032094/DK/NIDDK NIH HHS/United States
- R21 AR056128/AR/NIAMS NIH HHS/United States
- R21 EB003164/EB/NIBIB NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous