Glucocorticoids activate cardiac mineralocorticoid receptors in adrenalectomized Dahl salt-sensitive rats - PubMed (original) (raw)
. 2014 Feb;76(1-2):59-72.
- PMID: 25129992
- PMCID: PMC4345730
Glucocorticoids activate cardiac mineralocorticoid receptors in adrenalectomized Dahl salt-sensitive rats
Masafumi Ohtake et al. Nagoya J Med Sci. 2014 Feb.
Abstract
We previously showed that selective mineralocorticoid receptor (MR) blockade by eplerenone is cardioprotective in Dahl salt-sensitive (DS) rats. To clarify the consequences of glucocorticoid-mediated MR activation in these animals, we investigated the effects of exogenous corticosterone on blood pressure as well as cardiac remodeling and function after adrenalectomy. DS rats were subjected to adrenalectomy at 6 weeks of age and thereafter fed a high-salt diet and administered corticosterone (20 mg/kg per day) or vehicle. Systolic blood pressure was higher in the corticosterone group than in the vehicle group at 7 weeks and thereafter. By 11 weeks, corticosterone had reduced left ventricular (LV) mass and induced LV diastolic dysfunction. The ratio of collagen type I to type III mRNA levels in the left ventricle was increased in the corticosterone group compared with the vehicle group. Administration of a non-antihypertensive dose of the MR antagonist spironolactone (20 mg/kg per day) from 6 weeks inhibited the effects of corticosterone on both the collagen type I to type III mRNA ratio and diastolic function without affecting the decrease in LV mass. Spironolactone attenuated both the increase in NADPH oxidase activity in the left ventricle and coronary vascular inflammatory responses apparent in the corticosterone group. These results indicate that exogenous glucocorticoids induce hypertension, cardiac remodeling, and diastolic dysfunction in adrenalectomized DS rats fed a high-salt diet. The cardiac effects of exogenous glucocorticoids are likely attributable, at least in part, to myocardial oxidative stress and coronary vascular inflammation induced by glucocorticoid-activated MRs.
Figures
Fig. 1
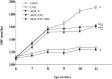
Plasma renin activity and aldosterone concentration in DS rats of the five experimental groups at 11 weeks of age. Renin activity (A) and aldosterone concentration (B) are presented as means ± SEM for animals in each group (n = 8, 7, 10, 10, and 9 for CONT, LVH, ADX+V, ADX+CTC, and ADX+CTC+SPL groups, respectively). *P < 0.05 versus CONT group; †P < 0.05 versus LVH group.
Fig. 2
Time course of SBP in DS rats of the five experimental groups. Data are means ± SEM for animals in each group. *P < 0.05 versus CONT group; †P < 0.05 versus LVH group; ‡P < 0.05 versus ADX+V group.
Fig. 3
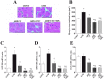
Cardiomyocyte size and expression of ANP, BNP, and IGF-1 genes in the left ventricle of DS rats in the five experimental groups at 11 weeks of age. (A) Hematoxylin-eosin staining of transverse sections of the LV myocardium. Scale bars, 50 µm. (B) Cross-sectional area of cardiac myocytes determined from sections similar to those in (A). (C–E) Quantitative RT-PCR analysis of ANP, BNP, and IGF-1 mRNAs, respectively. The amount of each mRNA was normalized by that of 18S rRNA and then expressed relative to the corresponding mean value for the CONT group. Data in (B) through (D) are means ± SEM for animals in each group. *P < 0.05 versus CONT group; †P < 0.05 versus LVH group; ‡P < 0.05 versus ADX+V group.
Fig. 4
Cardiac fibrosis and expression of collagen genes in the left ventricle of DS rats in the five experimental groups at 11 weeks of age. (A) Collagen deposition as revealed by Azan-Mallory staining in perivascular (upper panels) or interstitial (lower panels) regions of the LV myocardium. Scale bars, 200 µm. (B, C) Relative extents of perivascular and interstitial fibrosis, respectively, in the LV myocardium as determined from sections similar to those in (A). (D) Ratio of the amount of collagen type I mRNA to that of collagen type III mRNA. Data in (B) through (D) are means ± SEM for animals in each group. *P < 0.05 versus CONT group; †P < 0.05 versus LVH group; ‡P < 0.05 versus ADX+V group; §P < 0.05 versus ADX+CTC group.
Fig. 5
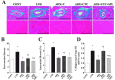
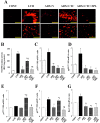
Superoxide production as well as NADPH oxidase activity and gene expression in the left ventricle of rats in the five experimental groups at 11 weeks of age. (A) Superoxide production as revealed by dihydroethidium staining in perivascular (upper panels) or interstitial (lower panels) regions of the LV myocardium. Scale bars, 100 µm. (B) NADPH-dependent superoxide production in LV homogenates. Results are expressed as relative light units (RLU) per milligram of protein. (C–G) Quantitative RT-PCR analysis of p22phox, gp91phox, p47phox, p67phox, and Rac1 mRNAs, respectively. The amount of each mRNA was normalized by that of 18S rRNA and then expressed relative to the corresponding mean value for the CONT group. Data in (B) through (G) are means ± SEM for animals in each group. *P < 0.05 versus CONT group; †P < 0.05 versus LVH group; ‡P < 0.05 versus ADX+V group; §P < 0.05 versus ADX+CTC group.
Fig. 6
Macrophage infiltration as well as expression of MCP-1, osteopontin, and COX-2 genes in the left ventricle of rats in the five experimental groups at 11 weeks of age. (A) Immunohistochemical staining for the monocyte-macrophage marker CD68. Scale bars, 50 µm. (B–D) Quantitative RT-PCR analysis of MCP-1, osteopontin, and COX-2 mRNAs, respectively. The amount of each mRNA was normalized by that of 18S rRNA and then expressed relative to the corresponding mean value for the CONT group. Data in (B) through (D) are means ± SEM for animals in each group. *P < 0.05 versus CONT group; †P < 0.05 versus LVH group; ‡P < 0.05 versus ADX+V group; §P < 0.05 versus ADX+CTC group.
Similar articles
- Glucocorticoid-induced hypertension and cardiac injury: effects of mineralocorticoid and glucocorticoid receptor antagonism.
Hattori T, Murase T, Iwase E, Takahashi K, Ohtake M, Tsuboi K, Ohtake M, Miyachi M, Murohara T, Nagata K. Hattori T, et al. Nagoya J Med Sci. 2013 Feb;75(1-2):81-92. Nagoya J Med Sci. 2013. PMID: 23544271 Free PMC article. - Mineralocorticoid receptor antagonism attenuates cardiac hypertrophy and failure in low-aldosterone hypertensive rats.
Nagata K, Obata K, Xu J, Ichihara S, Noda A, Kimata H, Kato T, Izawa H, Murohara T, Yokota M. Nagata K, et al. Hypertension. 2006 Apr;47(4):656-64. doi: 10.1161/01.HYP.0000203772.78696.67. Epub 2006 Feb 27. Hypertension. 2006. PMID: 16505208 - Mineralocorticoid receptor blockade attenuates chronic overexpression of the renin-angiotensin-aldosterone system stimulation of reduced nicotinamide adenine dinucleotide phosphate oxidase and cardiac remodeling.
Stas S, Whaley-Connell A, Habibi J, Appesh L, Hayden MR, Karuparthi PR, Qazi M, Morris EM, Cooper SA, Link CD, Stump C, Hay M, Ferrario C, Sowers JR. Stas S, et al. Endocrinology. 2007 Aug;148(8):3773-80. doi: 10.1210/en.2006-1691. Epub 2007 May 10. Endocrinology. 2007. PMID: 17494996 - Role of mineralocorticoid action in the brain in salt-sensitive hypertension.
Oki K, Gomez-Sanchez EP, Gomez-Sanchez CE. Oki K, et al. Clin Exp Pharmacol Physiol. 2012 Jan;39(1):90-5. doi: 10.1111/j.1440-1681.2011.05538.x. Clin Exp Pharmacol Physiol. 2012. PMID: 21585422 Free PMC article. Review. - The mammalian mineralocorticoid receptor: tying down a promiscuous receptor.
Gomez-Sanchez EP. Gomez-Sanchez EP. Exp Physiol. 2010 Jan;95(1):13-8. doi: 10.1113/expphysiol.2008.045914. Epub 2009 Jul 31. Exp Physiol. 2010. PMID: 19648477 Free PMC article. Review.
Cited by
- Bioactive Candy: Effects of Licorice on the Cardiovascular System.
Deutch MR, Grimm D, Wehland M, Infanger M, Krüger M. Deutch MR, et al. Foods. 2019 Oct 14;8(10):495. doi: 10.3390/foods8100495. Foods. 2019. PMID: 31615045 Free PMC article. Review. - Maternal obesity in the ewe increases cardiac ventricular expression of glucocorticoid receptors, proinflammatory cytokines and fibrosis in adult male offspring.
Ghnenis AB, Odhiambo JF, McCormick RJ, Nathanielsz PW, Ford SP. Ghnenis AB, et al. PLoS One. 2017 Dec 21;12(12):e0189977. doi: 10.1371/journal.pone.0189977. eCollection 2017. PLoS One. 2017. PMID: 29267325 Free PMC article. - Genomic and rapid effects of aldosterone: what we know and do not know thus far.
Hermidorff MM, de Assis LV, Isoldi MC. Hermidorff MM, et al. Heart Fail Rev. 2017 Jan;22(1):65-89. doi: 10.1007/s10741-016-9591-2. Heart Fail Rev. 2017. PMID: 27942913 Review. - Voluntary liquorice ingestion increases blood pressure via increased volume load, elevated peripheral arterial resistance, and decreased aortic compliance.
Hautaniemi EJ, Tahvanainen AM, Koskela JK, Tikkakoski AJ, Kähönen M, Uitto M, Sipilä K, Niemelä O, Mustonen J, Pörsti IH. Hautaniemi EJ, et al. Sci Rep. 2017 Sep 8;7(1):10947. doi: 10.1038/s41598-017-11468-7. Sci Rep. 2017. PMID: 28887501 Free PMC article. Clinical Trial. - Xiao-Qing-Long-Tang Maintains Cardiac Function during Heart Failure with Reduced Ejection Fraction in Salt-Sensitive Rats by Regulating the Imbalance of Cardiac Sympathetic Innervation.
Li Z, Wang Y, Jiang Y, Ma D, Jiang P, Zhou G, Yang J, Dong F, Zhao H, Zhang Y, Li X. Li Z, et al. Evid Based Complement Alternat Med. 2020 Nov 24;2020:9467271. doi: 10.1155/2020/9467271. eCollection 2020. Evid Based Complement Alternat Med. 2020. PMID: 33628295 Free PMC article.
References
- Muto T, Ueda N, Opthof T, Ohkusa T, Nagata K, Suzuki S, Tsuji Y, Horiba M, Lee JK, Honjo H, Kamiya K, Kodama I, Yasui K. Aldosterone modulates I(f) current through gene expression in cultured neonatal rat ventricular myocytes. Am J Physiol Heart Circ Physiol. 2007; 293: H2710–2718. - PubMed
- Rocha R, Rudolph AE, Frierdich GE, Nachowiak DA, Kekec BK, Blomme EA, McMahon EG, Delyani JA. Aldosterone induces a vascular inflammatory phenotype in the rat heart. Am J Physiol Heart Circ Physiol. 2002; 283: H1802–1810. - PubMed
- Qin W, Rudolph AE, Bond BR, Rocha R, Blomme EA, Goellner JJ, Funder JW, McMahon EG. Transgenic model of aldosterone-driven cardiac hypertrophy and heart failure. Circ Res. 2003; 93: 69–76. - PubMed
- Nagata K, Obata K, Xu J, Ichihara S, Noda A, Kimata H, Kato T, Izawa H, Murohara T, Yokota M. Mineralocorticoid receptor antagonism attenuates cardiac hypertrophy and failure in low-aldosterone hypertensive rats. Hypertension. 2006; 47: 656–664. - PubMed
- Nagata K. Mineralocorticoid antagonism and cardiac hypertrophy. Curr Hypertens Rep. 2008; 10: 216–221. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical