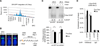A macrohistone variant links dynamic chromatin compaction to BRCA1-dependent genome maintenance - PubMed (original) (raw)
A macrohistone variant links dynamic chromatin compaction to BRCA1-dependent genome maintenance
Simran Khurana et al. Cell Rep. 2014.
Abstract
Appropriate DNA double-strand break (DSB) repair factor choice is essential for ensuring accurate repair outcome and genomic integrity. The factors that regulate this process remain poorly understood. Here, we identify two repressive chromatin components, the macrohistone variant macroH2A1 and the H3K9 methyltransferase and tumor suppressor PRDM2, which together direct the choice between the antagonistic DSB repair mediators BRCA1 and 53BP1. The macroH2A1/PRDM2 module mediates an unexpected shift from accessible to condensed chromatin that requires the ataxia telangiectasia mutated (ATM)-dependent accumulation of both proteins at DSBs in order to promote DSB-flanking H3K9 dimethylation. Remarkably, loss of macroH2A1 or PRDM2, as well as experimentally induced chromatin decondensation, impairs the retention of BRCA1, but not 53BP1, at DSBs. As a result, macroH2A1 and/or PRDM2 depletion causes epistatic defects in DSB end resection, homology-directed repair, and the resistance to poly(ADP-ribose) polymerase (PARP) inhibition-all hallmarks of BRCA1-deficient tumors. Together, these findings identify dynamic, DSB-associated chromatin reorganization as a critical modulator of BRCA1-dependent genome maintenance.
Copyright © 2014 The Authors. Published by Elsevier Inc. All rights reserved.
Figures
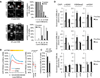
Figure 1. RNAi Screen Identifies a Role for MacroH2A1/MacroH2A1.2 in HR
(A) HR efficiency in DRGFP-U2OS cells stably transduced with shRNAs from a chromatin-focused RNAi library. HR was measured as percent (%) GFP+ cells; green diamonds represent macroH2A1-specific shRNAs. (B) HR efficiency (percent [%] GFP+ cells) in a Dox-inducible DRGFP gene conversion assay (see E). Samples were analyzed in triplicate. Values are expressed as mean and SD. Unless noted otherwise, p values are based on Student’s two-tailed t test: *p < 0.05; **p < 0.01; ***p < 0.001. (C) MacroH2A1.1-and macroH2A1.2-encoding mRNA levels of samples in (B) relative to RPL13a. Samples were analyzed in triplicate. Values are expressed as mean and SD. (D) Western blot analysis of macroH2A1 expression following macroH2A1 or macroH2A1.2 knockdown. (E) ChIP analysis 8 hr after release from double-thymidine block in the presence or absence of Dox-induced I-SceI expression. I-SceI DSB site-flanking primer locations are indicated (DSB site 1). A non-DSB-associated genomic locus served as control. Enrichment was normalized to no Dox. Values are expressed as mean and SEM (n ≥ 5). (F) Immunofluorescence analysis of macroH2A1.2 (top) or H3K9me2 (bottom) at laser-induced DSBs. Arrows depict the site of laser microirradiation; γ-H2AX served as a marker for DSBs. Arrows depict site of laser microirradiation. Scale bars, 10 µm. DSB-associated intensity changes were measured as the ratio of γ-H2AX+ over γ-H2AX− nuclear areas (7–18 cells per time point). Values are expressed as mean and SEM (n ≥ 3). R2 values are based on a third-order polynomial regression. See also Figures S1-S3 and Table S1.
Figure 2. PRDM2 Is a MacroH2A1.2-Dependent Regulator of HR
(A) Frequency of cells with H3K9me2 enrichment at laser-induced DSBs at the indicated time points after laser microirradiation. Values are expressed as mean and SEM (n = 4). (B) ChIP analysis 8 hr after release from double-thymidine block in the presence or absence of Dox. Enrichment relative to input is shown at the I-SceI DSB site and a non-DSB control locus. Values are expressed as mean and SEM (n = 3). (C) HR efficiency and PRDM2 mRNA levels following PRDM2 knockdown. Samples were analyzed in triplicate. mRNA levels are relative to sh-RFP and were normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH), RPL13a, and RPS16. Values are expressed as mean and SD. (D) ChIP analysis 8 hr after release from double-thymidine block in the presence or absence of Dox. Enrichment is shown relative to input. Values are expressed as mean and SEM (n = 3). (E) GFP-PRDM2 recruitment to laser-induced DSBs in cells expressing si-control (n = 29) or si-macroH2A1.2 (n = 30). Representative images are shown. Scale bars, 10 µm. Two independent experiments were combined. Data sets were subjected to Student’s two-tailed t test at each imaging time point. The p(mH2A1.2) heatmap depicts the p value distribution over time. gray indicates nonsignificance (ns). The right panel shows a representative box plot for data sets acquired 100 s post DSB. The red line indicates the median. The box shows the 25th–75th percentile. Whiskers show range between minimum and maximum values. (F and G) HR efficiency (F) and mRNA levels normalized to GAPDH, RPL13a, and RPS16 (G) in the presence of the indicated siRNAs. si-DKD, combined knockdown of macroH2A1.2 and PRDM2. Samples were analyzed in triplicate. Values are expressed as mean and SD. See also Figures S1, S4, and S5 and Movie S1.
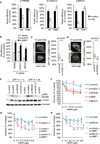
Figure 3. MacroH2A1, PRDM2, and H3K9me2 Accumulation at DSBs Is Dependent on ATM Kinase
(A) Coimmunostaining for macroH2A1.2 and γ-H2AX in the presence or absence of ATMi. Scale bars, 20 µm. The frequency of depletion (red arrows) or enrichment (white arrows) is shown for the indicated time points post laser microirradiation. (B) GFP-PRDM2 recruitment to sites of laser-induced DSBs in the absence (n = 60) or presence (n = 26) of ATMi. At least three independent experiments were combined. p(ATMi) heatmap and box plot were generated as described in Figure 2E. (C) Frequency of cells with laser damage-associated H3K9me2 in the presence or absence of ATMi (n = 3). (D) ChIP analysis 8 hr after release from double-thymidine block in the presence or absence of Dox. Enrichment relative to no Dox is shown for DSB site 1, DSB site 2 (see Figure 4B), and a non-DSB control locus. Values are expressed as mean and SEM (n = 3).
Figure 4. MacroH2A1 Promotes DSB-Induced DNase I Resistance
(A) 4C mapping of the I-SceI DSB site-containing DRGFP transgene. Normalized read counts are shown. Red and blue graphs represent independent experiments; bins are in 50 kb intervals. (B) Validation of the DRGFP integration site by DNA FISH. Green indicates GRIN2B–proximal BAC, and red indicates DRGFP probe. Scale bars, 1 mm. Colocalization was observed in ~25% of GRIN2B alleles, consistent with U2OS cell polyploidy. (C) Southern blot analysis of an endogenous DHS downstream of DRGFP. DHSR, DNase resistant; DHSS, DNase sensitive. A representative experiment is shown. DNase hypersensitivity was determined as the ratio of DHSS over total (DHSR + DHSS) signal intensities in the presence or absence of Dox. Values are expressed as mean and SEM (n = 3). (D) ChIP analysis 8 hr after release from double-thymidine block in the presence or absence of Dox. Enrichment relative to input is shown for the DSB-proximal DHS (DSB site 2). Values are expressed as mean and SEM (n = 3). See also Table S4.
Figure 5. MacroH2A1 and PRDM2 Promote ATM-Dependent Chromatin Condensation
(A) PAGFP-H2B imaging at the indicated time points after laser microirradiation. White lines depict maximal expansion (90 s). Scale bar, 10 µm. (B) Integrated PAGFP-H2B signal intensities normalized to t = 90 s. (C) Change in PAGFP-H2B nuclear area following laser microirradiation in sh-macroH2A1 (n = 39), sh-PRDM2–2 (n = 29), or sh-RFP cells (n = 32). Three independent experiments were combined. p(mH2A1) and p(PRDM2) heatmaps and box plot were generated as described in Figure 2E. (D) Change in PAGFP-H2B nuclear area in control (n = 42) and ATMi-treated cells (n = 39). Three independent experiments were pooled and analyzed as in (C). (E) Maximal expansion of PAGFP-H2B nuclear area following laser microirradiation in control (n = 36) and ATMi- (n = 35), or control (n = 47) and PARPi-treated cells (n =30). Nuclear area changes were normalized to the mean of controls. See also Movies S2 and S3.
Figure 6. Depletion of MacroH2A1/PRDM2 and Chromatin Decondensation Promote BRCA1 Loss at DSBs
(A) ChIP analysis 8 hr after release from double-thymidine block in the presence or absence of Dox. Enrichment relative to input is shown. Values are expressed as mean and SEM (n = 3). (B and C) Recruitment kinetics of GFP-BRCA1 (B) or GFP-53BP1 (C) to laser-induced DSBs in si-macroH2A1.2, si-PRDM2, and si-control cells. Two independent experiments were combined (n > 50 cells per sample). p(mH2A1.2) and p(PRDM2) heatmaps and box plot were generated as described in Figure 2E. (D) GFP-BRCA1 recruitment in the absence (n = 56) or presence of TSA (n = 50). At least three independent experiments were pooled and analyzed as described in (B). (E) GFP-53BP1 recruitment in the absence (n = 22) or presence of TSA (n = 31). Two independent experiments were combined and analyzed as in (B). (F) Peptide immunoprecipitation (IP) assays of HA-BRCA1 and HA-BARD1 with modified or unmodified histone H3 N-terminal peptides or beads alone (−). H3K9 peptide IPs were normalized to H3K9me2, H3K4 peptide IPs to the unmodified peptide (U). Values are expressed as mean and SEM (n ≥ 3). See also Figure S6.
Figure 7. MacroH2A1.2 and PRDM2 Direct Repair Pathway Choice by Promoting End Resection
(A) HR and NHEJ efficiency in stable U2OS reporter cell lines. Repair efficiency was normalized to si-control (black). Samples were analyzed in triplicate. Values are expressed as mean and SD. (B) HR efficiency in the presence or absence of si-53BP1. Samples were analyzed in triplicate. Values are expressed as mean and SD. (C) GFP-CtIP recruitment in S phase cells (1–2 hr post double-thymidine block). MacroH2A1.2 knockdown (n = 32) and control cells (n = 34) were analyzed 10 min post DSB. Two independent experiments were combined. Representative images are shown. Scale bars, 10 µm. (D) GFP-CtIP recruitment in sh-PRDM2–1 (n = 47) and sh-RFP control cells (n = 55). Three independent experiments were combined and analyzed as in (C). Representative images are shown. Scale bars, 10 µm. (E) Western blot analysis in U2OS cells treated with CPT for 1 hr followed by a 1 or 3 hr release. si-DKD, combined knockdown of macroH2A1.2 and PRDM2. (F–H) Clonogenic survival assays in response to treatment with PARPi. Samples were analyzed in triplicate. Values are expressed as mean and SD (Fand G) or as mean and SEM (n = 2) (H). See also Figure S7.
Comment in
- Chromatin yo-yo: expansion and condensation during DNA repair.
Li ML, Yuan G, Greenberg RA. Li ML, et al. Trends Cell Biol. 2014 Nov;24(11):616-618. doi: 10.1016/j.tcb.2014.09.004. Epub 2014 Oct 7. Trends Cell Biol. 2014. PMID: 25305135 Free PMC article.
Similar articles
- REV7 counteracts DNA double-strand break resection and affects PARP inhibition.
Xu G, Chapman JR, Brandsma I, Yuan J, Mistrik M, Bouwman P, Bartkova J, Gogola E, Warmerdam D, Barazas M, Jaspers JE, Watanabe K, Pieterse M, Kersbergen A, Sol W, Celie PHN, Schouten PC, van den Broek B, Salman A, Nieuwland M, de Rink I, de Ronde J, Jalink K, Boulton SJ, Chen J, van Gent DC, Bartek J, Jonkers J, Borst P, Rottenberg S. Xu G, et al. Nature. 2015 May 28;521(7553):541-544. doi: 10.1038/nature14328. Epub 2015 Mar 23. Nature. 2015. PMID: 25799992 Free PMC article. - DNA double-strand breaks promote methylation of histone H3 on lysine 9 and transient formation of repressive chromatin.
Ayrapetov MK, Gursoy-Yuzugullu O, Xu C, Xu Y, Price BD. Ayrapetov MK, et al. Proc Natl Acad Sci U S A. 2014 Jun 24;111(25):9169-74. doi: 10.1073/pnas.1403565111. Epub 2014 Jun 9. Proc Natl Acad Sci U S A. 2014. PMID: 24927542 Free PMC article. - ATM and CDK2 control chromatin remodeler CSB to inhibit RIF1 in DSB repair pathway choice.
Batenburg NL, Walker JR, Noordermeer SM, Moatti N, Durocher D, Zhu XD. Batenburg NL, et al. Nat Commun. 2017 Dec 4;8(1):1921. doi: 10.1038/s41467-017-02114-x. Nat Commun. 2017. PMID: 29203878 Free PMC article. - The influence of heterochromatin on DNA double strand break repair: Getting the strong, silent type to relax.
Goodarzi AA, Jeggo P, Lobrich M. Goodarzi AA, et al. DNA Repair (Amst). 2010 Dec 10;9(12):1273-82. doi: 10.1016/j.dnarep.2010.09.013. Epub 2010 Oct 30. DNA Repair (Amst). 2010. PMID: 21036673 Review. - Human syndromes with genomic instability and multiprotein machines that repair DNA double-strand breaks.
De la Torre C, Pincheira J, López-Sáez JF. De la Torre C, et al. Histol Histopathol. 2003 Jan;18(1):225-43. doi: 10.14670/HH-18.225. Histol Histopathol. 2003. PMID: 12507302 Review.
Cited by
- Histone H2A variants: Diversifying chromatin to ensure genome integrity.
Oberdoerffer P, Miller KM. Oberdoerffer P, et al. Semin Cell Dev Biol. 2023 Feb 15;135:59-72. doi: 10.1016/j.semcdb.2022.03.011. Epub 2022 Mar 21. Semin Cell Dev Biol. 2023. PMID: 35331626 Free PMC article. Review. - Immediate-Early, Early, and Late Responses to DNA Double Stranded Breaks.
Kieffer SR, Lowndes NF. Kieffer SR, et al. Front Genet. 2022 Jan 31;13:793884. doi: 10.3389/fgene.2022.793884. eCollection 2022. Front Genet. 2022. PMID: 35173769 Free PMC article. Review. - Comprehensive Mapping of Histone Modifications at DNA Double-Strand Breaks Deciphers Repair Pathway Chromatin Signatures.
Clouaire T, Rocher V, Lashgari A, Arnould C, Aguirrebengoa M, Biernacka A, Skrzypczak M, Aymard F, Fongang B, Dojer N, Iacovoni JS, Rowicka M, Ginalski K, Côté J, Legube G. Clouaire T, et al. Mol Cell. 2018 Oct 18;72(2):250-262.e6. doi: 10.1016/j.molcel.2018.08.020. Epub 2018 Sep 27. Mol Cell. 2018. PMID: 30270107 Free PMC article. - Joining the PARty: PARP Regulation of KDM5A during DNA Repair (and Transcription?).
Sanchez A, Buck-Koehntop BA, Miller KM. Sanchez A, et al. Bioessays. 2022 Jul;44(7):e2200015. doi: 10.1002/bies.202200015. Epub 2022 May 9. Bioessays. 2022. PMID: 35532219 Free PMC article. Review. - The Dynamic Behavior of Chromatin in Response to DNA Double-Strand Breaks.
García Fernández F, Fabre E. García Fernández F, et al. Genes (Basel). 2022 Jan 25;13(2):215. doi: 10.3390/genes13020215. Genes (Basel). 2022. PMID: 35205260 Free PMC article. Review.
References
- Altmeyer M, Lukas J. Guarding against collateral damage during chromatin transactions. Cell. 2013;153:1431–1434. - PubMed
- Altmeyer M, Toledo L, Gudjonsson T, Grøfte M, Rask MB, Lukas C, Akimov V, Blagoev B, Bartek J, Lukas J. The chromatin scaffold protein SAFB1 renders chromatin permissive for DNA damage signaling. Mol. Cell. 2013;52:206–220. - PubMed
- Ayoub N, Jeyasekharan AD, Bernal JA, Venkitaraman AR. HP1-beta mobilization promotes chromatin changes that initiate the DNA damage response. Nature. 2008;453:682–686. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous