The epigenetic landscape of alcoholism - PubMed (original) (raw)
Review
The epigenetic landscape of alcoholism
Harish R Krishnan et al. Int Rev Neurobiol. 2014.
Abstract
Alcoholism is a complex psychiatric disorder that has a multifactorial etiology. Epigenetic mechanisms are uniquely capable of accounting for the multifactorial nature of the disease in that they are highly stable and are affected by environmental factors, including alcohol itself. Chromatin remodeling causes changes in gene expression in specific brain regions contributing to the endophenotypes of alcoholism such as tolerance and dependence. The epigenetic mechanisms that regulate changes in gene expression observed in addictive behaviors respond not only to alcohol exposure but also to comorbid psychopathology such as the presence of anxiety and stress. This review summarizes recent developments in epigenetic research that may play a role in alcoholism. We propose that pharmacologically manipulating epigenetic targets, as demonstrated in various preclinical models, hold great therapeutic potential in the treatment and prevention of alcoholism.
Keywords: Alcoholism; Amygdala; Anxiety; DNA methylation; Histone acetylation; Negative affective state; Synaptic plasticity.
© 2014 Elsevier Inc. All rights reserved.
Figures
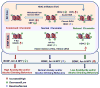
Figure 1
Schematic representation of the epigenetic mechanisms of acetylation and methylation of lysine (K) residues at histone H3 and H4 tails that mediate the switching between ‘open’ (relaxed) and ‘closed’ (condensed) chromatin structures. Acetylation of histone H3 at K9, K14, K18 and K56 and of H4 at K5, K8, K12 and K16 are believed to relax the chromatin structure making it more accessible to transcription factors and chromatin remodelers, thereby promoting transcription, whereas methylation at these lysine residues condenses the chromatin followed by reduced gene transcription. Histone deacetylases (HDACs) remove acetyl groups and histone methyltransferases (HMTs) attach methyl groups at lysine residues. HDACs are classified into four different classes (class I–IV), of which the class III, sirtuins, are NAD+ dependent and comprise of seven different isoforms (SIRT 1–7). HDACs classes I (HDAC 1, 2, 3, and 8), II (HDAC 4–7, 9, and 10) and IV (HDAC11) are Zn+ dependent enzymes. Histone methyltransferases such as G9a, GLP and SETDB1 can catalyze the methylation of H3-K9 contributing to the restrictive state of the chromatin. Histone demethylases (HDMs) (e.g. KDM family) remove the methyl groups from lysine residues associated with repressive marks (e.g. H3K9) in the tails of histones facilitating the relaxation of chromatin. Histone acetyltransferases (HATs) such as GNAT and MYST families of enzymes and CREB-binding protein (CBP)-p300 complex transfer acetyl groups leading to relaxed chromatin (Allis et al., 2007; Kouzarides, 2007).
Figure 2
Schematic representation of the histone acetylation mechanisms in the amygdala of Sprague-Dawley (SD) and alcohol preferring (P) and –non-preferring (NP) rats, that are operative in the modulation of ethanol drinking and anxiety-like behaviors. Acute ethanol exposure of SD rats inhibits the histone deacetylase (HDAC) activity and increases CBP expression, which has intrinsic histone acetyltransferase activity, leading to the hyperacetylation of histones. This relaxes chromatin and increases expression of activity-regulated cytoskeleton-associated protein (Arc), brain-derived neurotrophic factor (BDNF) and neuropeptide Y (NPY) resulting in acute ethanol’s anxiolytic effects. On the contrary, withdrawal from chronic ethanol treatment increases HDAC activity and decreases CBP levels leading to hypoacetylation of histones and a condensed state of chromatin that reduces BDNF, Arc and NPY, which precipitate anxiety-like behaviors. Treatment with trichostatin A (TSA), a pan-HDAC inhibitor, in alcohol-withdrawn SD rats was able to inhibit HDAC activity thereby increasing histone acetylation and expression of Arc, BDNF and NPY and further attenuated the withdrawal-related anxiety-like behaviors. Similarly, innately higher HDAC activity and HDAC2 expression in the amygdala of P rats compared to NP rats is associated with hypoacetylation of histones and low levels of Arc, BDNF and NPY and high levels of anxiety-like and alcohol-drinking behaviors. Inhibition of HDAC activity by treatment with TSA or knockdown of HDAC2 mRNA levels by using HDAC2 siRNA was able to correct the deficits in innate hypoacetylation and expression of Arc, BDNF and NPY thereby attenuating the high anxiety-like behavior and alcohol-preference in P rats (Pandey, Ugale et al., 2008; Moonat et al., 2013; You et al., 2014; Sakharkar, Zhang, et al., 2014).
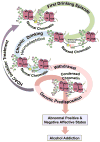
Figure 3
Hypothetical representation of chromatin remodeling driven by histone acetylation mechanisms in the amygdala that may be operative in the process of alcohol addiction. The first drinking episode (comparable to an acute ethanol treatment in animal models) may cause more acetylation of the histone tails due to HDAC inhibition that results in an ‘open’ or relaxed chromatin structure leading to euphoric effects e.g. anxiolysis. Repeated exposure to alcohol (chronic drinking) may lead to neuroadaptive changes in these epigenetic mechanisms translating into the development of chronic tolerance to these euphoric effects of ethanol. Consequentially, cessation from drinking may precipitate withdrawal-related anxiety due to a rebound phenomenon and underlying epigenetic causes, e.g. hypoacetylation due to an increase in HDAC activity further ‘closing’ or condensing the chromatin structure, which manifests in the negative affective state or the ‘dark side of addiction’ (Koob & Le Moal, 2005). This leads to the development of dysphoric symptoms such as anxiety and depression and subjects tend to self-medicate with ethanol to relieve these dysphoric symptoms, resulting in alcohol abuse and dependence. As seen from preclinical data (Figure 2), this cycle of withdrawal leading to self-medication can be broken with HDAC inhibitors, which by promoting histone acetylation can revert a ‘closed’ chromatin structure to an ‘open’ configuration and can lead to a normal behavioral state (Pandey, Ugale et al., 2008; Arora et al., 2013; Pandey, Ugale, et al., 2008; You et al., 2014). Innately condensed chromatin due to higher HDAC2 expression and deficits in histone acetylation in the amygdala may also be involved in anxiety and alcoholism (Moonat et al., 2013; Sakharkar, Zhang, et al., 2014).
Similar articles
- Epigenetic basis of the dark side of alcohol addiction.
Pandey SC, Kyzar EJ, Zhang H. Pandey SC, et al. Neuropharmacology. 2017 Aug 1;122:74-84. doi: 10.1016/j.neuropharm.2017.02.002. Epub 2017 Feb 4. Neuropharmacology. 2017. PMID: 28174112 Free PMC article. Review. - Aberrant histone deacetylase2-mediated histone modifications and synaptic plasticity in the amygdala predisposes to anxiety and alcoholism.
Moonat S, Sakharkar AJ, Zhang H, Tang L, Pandey SC. Moonat S, et al. Biol Psychiatry. 2013 Apr 15;73(8):763-73. doi: 10.1016/j.biopsych.2013.01.012. Epub 2013 Feb 26. Biol Psychiatry. 2013. PMID: 23485013 Free PMC article. - Epigenetics-beyond the genome in alcoholism.
Starkman BG, Sakharkar AJ, Pandey SC. Starkman BG, et al. Alcohol Res. 2012;34(3):293-305. Alcohol Res. 2012. PMID: 23134045 Free PMC article. - [Epigenetic mechanisms and alcohol use disorders: a potential therapeutic target].
Legastelois R, Jeanblanc J, Vilpoux C, Bourguet E, Naassila M. Legastelois R, et al. Biol Aujourdhui. 2017;211(1):83-91. doi: 10.1051/jbio/2017014. Epub 2017 Jul 6. Biol Aujourdhui. 2017. PMID: 28682229 Review. French. - Emerging Role of Epigenetic Mechanisms in Alcohol Addiction.
Berkel TD, Pandey SC. Berkel TD, et al. Alcohol Clin Exp Res. 2017 Apr;41(4):666-680. doi: 10.1111/acer.13338. Epub 2017 Feb 18. Alcohol Clin Exp Res. 2017. PMID: 28111764 Free PMC article. Review.
Cited by
- Histone Deacetylase Inhibitor Suberanilohydroxamic Acid Treatment Reverses Hyposensitivity to γ-Aminobutyric Acid in the Ventral Tegmental Area During Ethanol Withdrawal.
You C, Vandegrift BJ, Zhang H, Lasek AW, Pandey SC, Brodie MS. You C, et al. Alcohol Clin Exp Res. 2018 Nov;42(11):2160-2171. doi: 10.1111/acer.13870. Epub 2018 Sep 7. Alcohol Clin Exp Res. 2018. PMID: 30103280 Free PMC article. - Essential Role of Histone Methyltransferase G9a in Rapid Tolerance to the Anxiolytic Effects of Ethanol.
Berkel TDM, Zhang H, Teppen T, Sakharkar AJ, Pandey SC. Berkel TDM, et al. Int J Neuropsychopharmacol. 2019 Apr 1;22(4):292-302. doi: 10.1093/ijnp/pyy102. Int J Neuropsychopharmacol. 2019. PMID: 30590608 Free PMC article. - Effects of alcohol and PARP inhibition on RNA ribosomal engagement in cortical excitatory neurons.
Krishnan HR, Vallerini GP, Gavin HE, Guizzetti M, Rizavi HS, Gavin DP, Sharma RP. Krishnan HR, et al. Front Mol Neurosci. 2023 Apr 11;16:1125160. doi: 10.3389/fnmol.2023.1125160. eCollection 2023. Front Mol Neurosci. 2023. PMID: 37113267 Free PMC article. - "Boomerang Neuropathology" of Late-Onset Alzheimer's Disease is Shrouded in Harmful "BDDS": Breathing, Diet, Drinking, and Sleep During Aging.
Daulatzai MA. Daulatzai MA. Neurotox Res. 2015 Jul;28(1):55-93. doi: 10.1007/s12640-015-9528-x. Epub 2015 Apr 25. Neurotox Res. 2015. PMID: 25911292 Review. - Binge-Like Alcohol Exposure During Adolescence Disrupts Dopaminergic Neurotransmission in the Adult Prelimbic Cortex.
Trantham-Davidson H, Centanni SW, Garr SC, New NN, Mulholland PJ, Gass JT, Glover EJ, Floresco SB, Crews FT, Krishnan HR, Pandey SC, Chandler LJ. Trantham-Davidson H, et al. Neuropsychopharmacology. 2017 Apr;42(5):1024-1036. doi: 10.1038/npp.2016.190. Epub 2016 Sep 13. Neuropsychopharmacology. 2017. PMID: 27620551 Free PMC article.
References
- Alarcón JM, Malleret G, Touzani K, Vronskaya S, Ishii S, Kandel ER, Barco A. Chromatin acetylation, memory, and LTP are impaired in CBP+/− mice: a model for the cognitive deficit in Rubinstein-Taybi syndrome and its amelioration. Neuron. 2004;42(6):947–959. - PubMed
- Allegrucci C, Thurston A, Lucas E, Young L. Epigenetics and the germline. Reproduction. 2005;129(2):137–149. - PubMed
- Allis CD, Berger SL, Cote J, Dent S, Jenuwien T, Kouzarides T, Pillus L, Reinberg D, Shi Y, Shiekhattar R, Shilatifard A, Workman J, Zhang Y. New nomenclature for chromatin-modifying enzymes. Cell. 2007;131(4):633–636. - PubMed
- Amir RE, Van den Veyver IB, Wan M, Tran CQ, Francke U, Zoghbi HY. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat Genet. 1999;23(2):185–188. - PubMed
Publication types
MeSH terms
Grants and funding
- R29 AA010005/AA/NIAAA NIH HHS/United States
- U01 AA019971/AA/NIAAA NIH HHS/United States
- AA-019971/AA/NIAAA NIH HHS/United States
- AA-016690/AA/NIAAA NIH HHS/United States
- R01 AA010005/AA/NIAAA NIH HHS/United States
- R01 AA016690/AA/NIAAA NIH HHS/United States
- R01 AA013341/AA/NIAAA NIH HHS/United States
- AA-013341/AA/NIAAA NIH HHS/United States
- I01 BX000143/BX/BLRD VA/United States
- AA-010005/AA/NIAAA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical