The ubiquitin-proteasome system in neurodegenerative diseases: precipitating factor, yet part of the solution - PubMed (original) (raw)
Review
The ubiquitin-proteasome system in neurodegenerative diseases: precipitating factor, yet part of the solution
Nico P Dantuma et al. Front Mol Neurosci. 2014.
Abstract
The ubiquitin-proteasome system (UPS) has been implicated in neurodegenerative diseases based on the presence of deposits consisting of ubiquitylated proteins in affected neurons. It has been postulated that aggregation-prone proteins associated with these disorders, such as α-synuclein, β-amyloid peptide, and polyglutamine proteins, compromise UPS function, and delay the degradation of other proteasome substrates. Many of these substrates play important regulatory roles in signaling, cell cycle progression, or apoptosis, and their inadvertent stabilization due to an overloaded and improperly functioning UPS may thus be responsible for cellular demise in neurodegeneration. Over the past decade, numerous studies have addressed the UPS dysfunction hypothesis using various model systems and techniques that differ in their readout and sensitivity. While an inhibitory effect of some disease proteins on the UPS has been demonstrated, increasing evidence attests that the UPS remains operative in many disease models, which opens new possibilities for treatment. In this review, we will discuss the paradigm shift that repositioned the UPS from being a prime suspect in the pathophysiology of neurodegeneration to an attractive therapeutic target that can be harnessed to accelerate the clearance of disease-linked proteins.
Keywords: neurodegeneration; proteasome; protein quality control; proteolysis; ubiquitin.
Figures
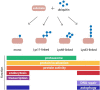
Figure 1
Structure and function of common ubiquitin modifications. Ubiquitin may be conjugated to protein substrates as either a monomer or a polymeric chain, in which one of seven internal lysine (Lys) residues of ubiquitin, or the N-terminal methionine, serves as an acceptor for additional ubiquitin moieties. The type of polyubiquitin linkage dictates the topology of the resulting chain. Ubiquitin modifications can regulate protein function or act as a signal in many cellular processes. Examples for functions of monoubiquitylation, and homogenous Lys11-, Lys48-, and Lys63-linked polyubiquitin chains are shown.
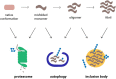
Figure 2
Cellular pathways that counteract protein aggregation are ubiquitin-dependent processes. Proteins linked to neurodegenerative diseases, such as α-synuclein, β-amyloid peptide and polyQ proteins, are prone to misfolding and aggregation in the cellular environment. The proteasome, autophagy, and inclusion bodies form a network of quality control systems which reduces levels of misfolded proteins and counteracts aggregation. All three pathways are regulated by ubiquitylation.
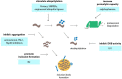
Figure 3
Targeting the ubiquitin-proteasome system (UPS) in neurodegenerative disorders using small molecules or engineering approaches. Various events in the UPS can be targeted by compounds in order to stimulate UPS activity. Among those events are accelerating of ubiquitylation by compounds or engineered ubiquitin ligases, inhibition of deubiquitylation, inhibition of protein aggregation so that the proteins remain in a state that is permissible to proteasomal degradation and stimulation of the formation of inclusion bodies which may reduce the load of aggregation-prone proteins and preserve UPS activity.
Similar articles
- The ubiquitin proteasome system in neurodegenerative diseases: culprit, accomplice or victim?
Dennissen FJ, Kholod N, van Leeuwen FW. Dennissen FJ, et al. Prog Neurobiol. 2012 Feb;96(2):190-207. doi: 10.1016/j.pneurobio.2012.01.003. Epub 2012 Jan 16. Prog Neurobiol. 2012. PMID: 22270043 Review. - Exploring the Promise of Targeting Ubiquitin-Proteasome System to Combat Alzheimer's Disease.
Al Mamun A, Uddin MS, Kabir MT, Khanum S, Sarwar MS, Mathew B, Rauf A, Ahmed M, Ashraf GM. Al Mamun A, et al. Neurotox Res. 2020 Jun;38(1):8-17. doi: 10.1007/s12640-020-00185-1. Epub 2020 Mar 9. Neurotox Res. 2020. PMID: 32157628 Review. - Targeting Ubiquitin-Proteasome Pathway by Natural Products: Novel Therapeutic Strategy for Treatment of Neurodegenerative Diseases.
Momtaz S, Memariani Z, El-Senduny FF, Sanadgol N, Golab F, Katebi M, Abdolghaffari AH, Farzaei MH, Abdollahi M. Momtaz S, et al. Front Physiol. 2020 Apr 28;11:361. doi: 10.3389/fphys.2020.00361. eCollection 2020. Front Physiol. 2020. PMID: 32411012 Free PMC article. - Natural alkaloid harmine promotes degradation of alpha-synuclein via PKA-mediated ubiquitin-proteasome system activation.
Cai CZ, Zhou HF, Yuan NN, Wu MY, Lee SM, Ren JY, Su HX, Lu JJ, Chen XP, Li M, Tan JQ, Lu JH. Cai CZ, et al. Phytomedicine. 2019 Aug;61:152842. doi: 10.1016/j.phymed.2019.152842. Epub 2019 Jan 30. Phytomedicine. 2019. PMID: 31048127 - Aim for the core: suitability of the ubiquitin-independent 20S proteasome as a drug target in neurodegeneration.
Opoku-Nsiah KA, Gestwicki JE. Opoku-Nsiah KA, et al. Transl Res. 2018 Aug;198:48-57. doi: 10.1016/j.trsl.2018.05.002. Epub 2018 Jun 19. Transl Res. 2018. PMID: 30244692 Free PMC article. Review.
Cited by
- Sirtuins and proteolytic systems: implications for pathogenesis of synucleinopathies.
Sampaio-Marques B, Ludovico P. Sampaio-Marques B, et al. Biomolecules. 2015 May 4;5(2):735-57. doi: 10.3390/biom5020735. Biomolecules. 2015. PMID: 25946078 Free PMC article. Review. - Prion degradation pathways: Potential for therapeutic intervention.
Goold R, McKinnon C, Tabrizi SJ. Goold R, et al. Mol Cell Neurosci. 2015 May;66(Pt A):12-20. doi: 10.1016/j.mcn.2014.12.009. Epub 2015 Jan 10. Mol Cell Neurosci. 2015. PMID: 25584786 Free PMC article. Review. - Protein citrullination marks myelin protein aggregation and disease progression in mouse ALS models.
Yusuf IO, Qiao T, Parsi S, Tilvawala R, Thompson PR, Xu Z. Yusuf IO, et al. Acta Neuropathol Commun. 2022 Sep 8;10(1):135. doi: 10.1186/s40478-022-01433-5. Acta Neuropathol Commun. 2022. PMID: 36076282 Free PMC article. - Hybrid Chains: A Collaboration of Ubiquitin and Ubiquitin-Like Modifiers Introducing Cross-Functionality to the Ubiquitin Code.
Pérez Berrocal DA, Witting KF, Ovaa H, Mulder MPC. Pérez Berrocal DA, et al. Front Chem. 2020 Jan 22;7:931. doi: 10.3389/fchem.2019.00931. eCollection 2019. Front Chem. 2020. PMID: 32039151 Free PMC article. Review. - Cross Talk of Proteostasis and Mitostasis in Cellular Homeodynamics, Ageing, and Disease.
Gumeni S, Trougakos IP. Gumeni S, et al. Oxid Med Cell Longev. 2016;2016:4587691. doi: 10.1155/2016/4587691. Epub 2016 Feb 9. Oxid Med Cell Longev. 2016. PMID: 26977249 Free PMC article. Review.
References
- Adachi H., Waza M., Tokui K., Katsuno M., Minamiyama M., Tanaka F., et al. (2007). CHIP overexpression reduces mutant androgen receptor protein and ameliorates phenotypes of the spinal and bulbar muscular atrophy transgenic mouse model. J. Neurosci. 27, 5115–5126 10.1523/JNEUROSCI.1242-07.2007 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous