Targeting the ubiquitin-proteasome system in heart disease: the basis for new therapeutic strategies - PubMed (original) (raw)
Review
Targeting the ubiquitin-proteasome system in heart disease: the basis for new therapeutic strategies
Oliver Drews et al. Antioxid Redox Signal. 2014.
Abstract
Significance: Novel therapeutic strategies to treat heart failure are greatly needed. The ubiquitin-proteasome system (UPS) affects the structure and function of cardiac cells through targeted degradation of signaling and structural proteins. This review discusses both beneficial and detrimental consequences of modulating the UPS in the heart.
Recent advances: Proteasome inhibitors were first used to test the role of the UPS in cardiac disease phenotypes, indicating therapeutic potential. In early cardiac remodeling and pathological hypertrophy with increased proteasome activities, proteasome inhibition prevented or restricted disease progression and contractile dysfunction. Conversely, enhancing proteasome activities by genetic manipulation, pharmacological intervention, or ischemic preconditioning also improved the outcome of cardiomyopathies and infarcted hearts with impaired cardiac and UPS function, which is, at least in part, caused by oxidative damage.
Critical issues: An understanding of the UPS status and the underlying mechanisms for its potential deregulation in cardiac disease is critical for targeted interventions. Several studies indicate that type and stage of cardiac disease influence the dynamics of UPS regulation in a nonlinear and multifactorial manner. Proteasome inhibitors targeting all proteasome complexes are associated with cardiotoxicity in humans. Furthermore, the type and dosage of proteasome inhibitor impact the pathogenesis in nonuniform ways.
Future directions: Systematic analysis and targeting of individual UPS components with established and innovative tools will unravel and discriminate regulatory mechanisms that contribute to and protect against the progression of cardiac disease. Integrating this knowledge in drug design may reduce adverse effects on the heart as observed in patients treated with proteasome inhibitors against noncardiac diseases, especially cancer.
Figures
**FIG. 1.
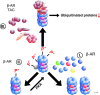
The UPS is a multilayered machinery. E1 enzymes activate, E2 conjugate, and E3 ligate ubiquitin proteins to target substrate proteins for proteasomal degradation by 26S proteasomes. If E4 enzymes aid in the process of ubiquitination, multiple ubiquitins are transferred in the form of ubiquitin chains (100). Ubiquitination is counteracted by DUBs, but deubiquitination at the 19S regulatory particle is also required for efficient degradation and ubiquitin recycling. The 26S proteasomes are composed of 19S regulatory and 20S core particles. Association of 19S with 20S proteasomes and substrate unfolding via the 19S proteasome are ATP dependent. The numbers in parentheses indicate the number of distinct genes encoding for proteins of the corresponding layer. It should be noted that not all UPS proteins are required simultaneously. A single E3 enzyme or 14 different 20S proteasome subunits are sufficient for ubiquitin ligation or a functional 20S proteasome, respectively. Furthermore, many E3 genes have been identified by homology only. To see this illustration in color, the reader is referred to the web version of this article at
**FIG. 2.
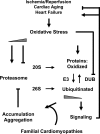
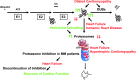
Proposed role for the ubiquitin-proteasome system in heart disease with focus on oxidative stress. In cardiac tissue, oxidative stress occurs under conditions, such as ischemia/reperfusion, aging, and heart failure (35, 118, 194). At low stress levels, such as ischemic preconditioning or early cardiac remodeling, proteasome function is adjusted presumably in a compensatory manner (5, 29, 40, 45, 49). Protein substrates of the UPS participate in cardio-protective as well as in pathologic signaling (e.g., cardioprotection/PKCɛ, pro-inflammatory/NF-κB, pro-hypertrophic/calcineurin; Table 1), but not all substrates are affected by E3 ligases, DUBs, and proteasomal degradation equally. Thus, targeting the UPS in cardiac disease will be dependent on type and stage of the pathogenesis. With increasing stress load, reduced proteasome function is observed while oxidized and ubiquitinated proteins accumulate (46, 169, 170, 219). In turn, aggregation of carbonylated and ubiquitinated proteins (181) as well as 4-hydroxy-2-nonenal modification and carbonylation of 20S and 19S proteasome subunits are considered to inhibit proteasome activities (21, 45, 170), forming a vicious cycle. Inhibition of DUBs due to oxidation promotes the degradation of proteasomal substrates (55, 126), but potentially contributes to protein aggregation in case of insufficient proteasome activities. Progressive oxidative damage may contribute to the higher incidence of cardiac disease with increasing age. Familial cardiomyopathies with a mutation in genes encoding proteasome substrates seem to accelerate the cycle by facilitating aggregate formation (7, 135, 182).
**FIG. 3.
Proteasomes are a heterogenic group of multi-protein complexes. Cardiac 20S proteasomes incorporate one or two of six different proteolytic subunits with distinct preference of cleavage sites and turnover and, hence, exist in subpopulations in mammalian hearts (50). Mixed assembly with constitutive and inducible proteolytic subunits results in the formation of intermediate subpopulations (…indicates that additional subpopulations exist). Any of these subpopulations can assemble with approximately two regulators (top and bottom panels) and the possibility of forming hybrid proteasome complexes (assembly with two different regulators) (26, 74). Post-translational modifications of proteasome subunits cause further heterogeneity (73, 138, 233). To see this illustration in color, the reader is referred to the web version of this article at
**FIG. 4.
Mechanisms for enhancing proteasome activities in the myocardium. Ischemic preconditioning preserves proteasome activities during subsequent ischemia/reperfusion treatments and is associated with reduced infarct size, decreased Bax, PKCδ, and PTEN abundance, as well as less accumulation of ubiquitinated and oxidized proteins (5, 23, 29, 45). Preservation and stimulation of 26S proteasome activities is at least, in part, mediated by PKA (5) and PKCɛ (29) and protection against reactive oxygen species (ROS) (45). *Stimulation of proteasome activities by PKA is observed in other models as well (49, 183, 230, 232). Cardiac expression of constitutive active PKN enhances 26S proteasome activity and assembly, which is associated with hypertrophy (197). It also protects against ischemia/reperfusion injury by reducing the infarct size. Overexpression of the 11S subunit PA28α increases the turnover of the proteasome reporter substrate GFP/CL1, potentially via increased 11S assembly with 26S proteasomes (132, 133). Genetic proteasome enhancement via PA28α reduces infarct size induced by cardiac ischemia/reperfusion as well (132). In a model of desmin-related cardiomyopathy, PA28α overexpression enhances lifespan and reduces hypertrophy, abundance of mutated CryAB aggregates, as well as ubiquitinated proteins (132). Similarly, decreased GFP/CL1 abundance after PKG activation via sildenafil is associated with reduced hypertrophy, less aggregation of mutated CryAB, and preserved cardiac function in the same DRC model (173). To see this illustration in color, the reader is referred to the web version of this article at
**FIG. 5.
Modulation of cardiac tissue mass via the UPS. In mice subjected to transverse aortic constriction (TAC), knock-out of the E3 ligase MuRF1 promotes cardiac hypertrophy (222). After TAC release, MuRF1 promotes cardiac atrophy in wild-type mice (223). MuRF1 also promotes cardiac atrophy in response to fasting (10). Atrogin-1 overexpression (OE) in the TAC model reduces hypertrophic remodeling (130). Administration of 20S proteasome inhibitors in the TAC model, after β-adrenoreceptor (β-AR) stimulation, or in hypertensive Dahl-salt sensitive rats (DSS), reduces hypertrophic remodeling (40, 85, 144, 193), promotes reverse remodeling (85, 193), and improves cardiac function (85). Modulation of cardiac tissue mass via deubiquitinating enzymes (DUBs) may be possible, but to our knowledge has not yet been reported. To see this illustration in color, the reader is referred to the web version of this article at
**FIG. 6.
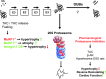
Proteasome regulation during hypertrophic remodeling. At least three mechanisms regulate proteasome activities during cardiac remodeling. I. After continuous β-adrenoreceptor stimulation (β-AR), the composition of 20S proteasome subpopulations is altered, due to increasing incorporation of inducible subunits during proteasome assembly, which changes the ratios of proteolytic activities (49). ‘…’ indicates that additional possibilities for subpopulation assembly exist. II. In addition, a decline in 20S proteasome activities occurs, which can be rescued by cAMP or PKA signaling. At the current state of investigations, it is unknown whether the rescue is mediated by phosphorylation (P−) of 20S proteasomes or an associating partner (depicted in gray, attached to the 20S proteasome). III. Increased 26S proteasome assembly and activities correlate with decreased abundance of ubiquitinated proteins in hypertrophic hearts after continuous β-AR stimulation (49) and transverse aortic constriction (TAC) (40). To see this illustration in color, the reader is referred to the web version of this article at
**FIG. 7.
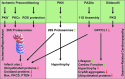
Coupling of cardiac function and the UPS in human cardiac disease. Several investigations report increased abundance of ubiquitinated proteins in human heart disease (87, 170, 205, 219, 225). This is paralleled by increased abundance of a deubiquitinating enzyme in dilated cardiomyopathy (219), decreased 20S proteasome subunit abundance in congestive heart failure (225), decreased assembly of 19S with 20S proteasomes (37), and decreased 26S proteasome activities in hypertrophic cardiomyopathy and heart failure (170). Ventricular unloading after implantation of left ventricular assist devices decreases the abundance of ubiquitinated proteins (112, 225), and stimulates the expression of proteasome subunits (225) as well as proteasome activities (112, 170). Therapeutic proteasome inhibition in patients with multiple myeloma (MM) is associated with the onset and deterioration of cardiac disease in several reports (15, 36, 56, 62, 81, 82, 198, 211), which is reversible after discontinuation of the therapy (15, 81, 82, 211). To see this illustration in color, the reader is referred to the web version of this article at
Similar articles
- Proteasome functional insufficiency in cardiac pathogenesis.
Wang X, Li J, Zheng H, Su H, Powell SR. Wang X, et al. Am J Physiol Heart Circ Physiol. 2011 Dec;301(6):H2207-19. doi: 10.1152/ajpheart.00714.2011. Epub 2011 Sep 23. Am J Physiol Heart Circ Physiol. 2011. PMID: 21949118 Free PMC article. Review. - The role of ubiquitin ligases in cardiac disease.
Willis MS, Bevilacqua A, Pulinilkunnil T, Kienesberger P, Tannu M, Patterson C. Willis MS, et al. J Mol Cell Cardiol. 2014 Jun;71:43-53. doi: 10.1016/j.yjmcc.2013.11.008. Epub 2013 Nov 19. J Mol Cell Cardiol. 2014. PMID: 24262338 Free PMC article. Review. - Proteasome biology and therapeutics in cardiac diseases.
Shukla SK, Rafiq K. Shukla SK, et al. Transl Res. 2019 Mar;205:64-76. doi: 10.1016/j.trsl.2018.09.003. Epub 2018 Sep 28. Transl Res. 2019. PMID: 30342797 Free PMC article. Review. - Into the heart: the emerging role of the ubiquitin-proteasome system.
Willis MS, Patterson C. Willis MS, et al. J Mol Cell Cardiol. 2006 Oct;41(4):567-79. doi: 10.1016/j.yjmcc.2006.07.015. Epub 2006 Sep 1. J Mol Cell Cardiol. 2006. PMID: 16949602 Review. - Perturbation of cullin deneddylation via conditional Csn8 ablation impairs the ubiquitin-proteasome system and causes cardiomyocyte necrosis and dilated cardiomyopathy in mice.
Su H, Li J, Menon S, Liu J, Kumarapeli AR, Wei N, Wang X. Su H, et al. Circ Res. 2011 Jan 7;108(1):40-50. doi: 10.1161/CIRCRESAHA.110.230607. Epub 2010 Nov 4. Circ Res. 2011. PMID: 21051661 Free PMC article.
Cited by
- RING finger protein 10 is a potential drug target for diabetic vascular complications.
Li S, Yu G, Huang W, Wang R, Pu P, Chen M. Li S, et al. Mol Med Rep. 2019 Aug;20(2):931-938. doi: 10.3892/mmr.2019.10358. Epub 2019 Jun 6. Mol Med Rep. 2019. PMID: 31173254 Free PMC article. - Genetic ablation of Lmp2 increases the susceptibility for impaired cardiac function.
Trogisch FA, Koser F, Michel S, Liem DA, Florea BI, Hecker M, Drews O. Trogisch FA, et al. Front Mol Biosci. 2024 Mar 7;11:1148948. doi: 10.3389/fmolb.2024.1148948. eCollection 2024. Front Mol Biosci. 2024. PMID: 38516190 Free PMC article. - Coordinating Regulation of Gene Expression in Cardiovascular Disease: Interactions between Chromatin Modifiers and Transcription Factors.
Bauer AJ, Martin KA. Bauer AJ, et al. Front Cardiovasc Med. 2017 Apr 6;4:19. doi: 10.3389/fcvm.2017.00019. eCollection 2017. Front Cardiovasc Med. 2017. PMID: 28428957 Free PMC article. Review. - Proteomic Analysis in Valvular Cardiomyopathy: Aortic Regurgitation vs. Aortic Stenosis.
Holst T, Petersen J, Ameling S, Müller L, Christ T, Gedeon N, Eschenhagen T, Reichenspurner H, Hammer E, Girdauskas E. Holst T, et al. Cells. 2023 Mar 11;12(6):878. doi: 10.3390/cells12060878. Cells. 2023. PMID: 36980219 Free PMC article. - Structure, Dynamics and Function of the 26S Proteasome.
Mao Y. Mao Y. Subcell Biochem. 2021;96:1-151. doi: 10.1007/978-3-030-58971-4_1. Subcell Biochem. 2021. PMID: 33252727 Review.
References
- Medical Review for NDA 21-602 VELCADE (bortezomib) for Injection. www.accessdata.fda.gov/drugsatfda_docs/nda/2003/21602_Velcade_medr.pdf
- Adams V, Linke A, Gielen S, Erbs S, Hambrecht R, and Schuler G. Modulation of Murf-1 and MAFbx expression in the myocardium by physical exercise training. Eur J Cardiovasc Prev Rehabil 15: 293–299, 2008 - PubMed
- Angeles A, Fung G, and Luo H. Immune and non-immune functions of the immunoproteasome. Front Biosci (Landmark Ed) 17: 1904–1916, 2012 - PubMed
- Asai M, Tsukamoto O, Minamino T, Asanuma H, Fujita M, Asano Y, Takahama H, Sasaki H, Higo S, Asakura M, Takashima S, Hori M, and Kitakaze M. PKA rapidly enhances proteasome assembly and activity in in vivo canine hearts. J Mol Cell Cardiol 46: 452–462, 2009 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical