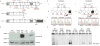Mycobacterium simiae infection in two unrelated patients with different forms of inherited IFN-γR2 deficiency - PubMed (original) (raw)
Case Reports
doi: 10.1007/s10875-014-0085-5. Epub 2014 Aug 19.
Orli Megged, Polina Stepensky, Pierre Casimir, Marcela Moncada-Velez, Diana Averbuch, Marc Victor Assous, Omar Abuzaitoun, Xiao-Fei Kong, Vincent Pedergnana, Caroline Deswarte, Mélanie Migaud, Stefan Rose-John, Yuval Itan, Bertrand Boisson, Aziz Belkadi, Francesca Conti, Laurent Abel, Guillaume Vogt, Stephanie Boisson-Dupuis, Jean-Laurent Casanova, Jacinta Bustamante
Affiliations
- PMID: 25135595
- PMCID: PMC4241769
- DOI: 10.1007/s10875-014-0085-5
Case Reports
Mycobacterium simiae infection in two unrelated patients with different forms of inherited IFN-γR2 deficiency
Rubén Martínez-Barricarte et al. J Clin Immunol. 2014 Nov.
Abstract
Interferon-γ receptor 2 (IFN-γR2) deficiency is a rare primary immunodeficiency characterized by predisposition to infections with weakly virulent mycobacteria, such as environmental mycobacteria and BCG vaccines. We describe here two children with IFN-γR2 deficiency, from unrelated, consanguineous kindreds of Arab and Israeli descent. The first patient was a boy who died at the age of 4.5 years, from recurrent, disseminated disease caused by Mycobacterium simiae. His IFN-γR2 defect was autosomal recessive and complete. The second patient was a girl with multiple disseminated mycobacterial infections, including infection with M. simiae. She died at the age of 5 years, a short time after the transplantation of umbilical cord blood cells from an unrelated donor. Her IFN-γR2 defect was autosomal recessive and partial. Autosomal recessive IFN-γR2 deficiency is life-threatening, even in its partial form, and genetic diagnosis and familial counseling are therefore particularly important for this condition. These two cases are the first of IFN-γR2 deficiency associated with M. simiae infection to be described.
Conflict of interest statement
Conflict of interest: The authors have no conflict of interest to declare.
Figures
Fig. 1. Loss-of-function IFNGR2 alleles associated with MSMD
a) Schematic representation of the human IFNGR2 gene and corresponding protein with all previously described MSMD-associated mutations: in blue, mutations causing AR complete IFN-γR2 deficiency with detectable surface expression; in red, AR IFN-γR2 deficiency with no receptor expression; in purple, AR partial IFN-γR2 deficiency, and in green, AD IFN-γR2 deficiency. The different domains of the protein are indicated as L (leader peptide), EC (extracellular domain), TM (transmembrane domain) and IC (intracellular domain). Below this diagram, the consequences of the two mutations described here are shown: in the middle panel, a short IFN-γR2 caused by the creation of a premature stop codon (Y179X) and at the bottom, a longer predicted protein caused by the c.958insT variant. The extra portion of the protein due to the frame shift is indicated at dark gray at the end of the diagram. b) Familial segregation of the two mutations (Y179X and c.958insT) found in IFNGR2. The index case (P1 and P2) of each pedigree is indicated with solid symbols and an arrow. Each kindred is designated by a capital letter (A-B), and each generation by a Roman numeral (I-II). c) Electropherograms of a wild-type homozygous individual, a heterozygous carrier and a homozygous carrier are shown in this panel, in which the position of the mutant form is indicated with an arrow. d) Overexpression of the WT and mutant alleles of IFNGR2 in IFN-γR2-deficient SV40-fibroblasts, revealing a lack of expression of Y179X and the residual expression of 958insT. The results presented are from one experiment representative of the three independent experiments carried out. e) EMSA was carried out after IFN-γ stimulation of the same transfected cells as in D. A complete absence of DNA binding was observed for the nonsense mutation. The frameshift insertion showed a partial DNA binding, which was comparable to a previously reported partial deficient mutation. The results shown are representative of five independent experiments.
Fig. 2. Plasma IFN-γ concentration
Plasma IFN-γ concentrations of the patients carrying the reported mutations. These concentrations are higher than those in healthy controls. The graph shows IFN-γ concentrations for the different forms of IFN-γR2 deficiency. The values obtained for these two patients (P1, P2) are similar to those reported for other patients with AR IFN-γR2 deficiency.
Similar articles
- Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.
Salisbury L, Baraitser L. Salisbury L, et al. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review. - A Novel Splice Site Mutation in IFNGR2 in Patients With Primary Immunodeficiency Exhibiting Susceptibility to Mycobacterial Diseases.
Bandari AK, Muthusamy B, Bhat S, Govindaraj P, Rajagopalan P, Dalvi A, Shankar S, Raja R, Reddy KS, Madkaikar M, Pandey A. Bandari AK, et al. Front Immunol. 2019 Aug 21;10:1964. doi: 10.3389/fimmu.2019.01964. eCollection 2019. Front Immunol. 2019. PMID: 31497017 Free PMC article. - Comparison of Two Modern Survival Prediction Tools, SORG-MLA and METSSS, in Patients With Symptomatic Long-bone Metastases Who Underwent Local Treatment With Surgery Followed by Radiotherapy and With Radiotherapy Alone.
Lee CC, Chen CW, Yen HK, Lin YP, Lai CY, Wang JL, Groot OQ, Janssen SJ, Schwab JH, Hsu FM, Lin WH. Lee CC, et al. Clin Orthop Relat Res. 2024 Dec 1;482(12):2193-2208. doi: 10.1097/CORR.0000000000003185. Epub 2024 Jul 23. Clin Orthop Relat Res. 2024. PMID: 39051924 - Genedrive kit for detecting single nucleotide polymorphism m.1555A>G in neonates and their mothers: a systematic review and cost-effectiveness analysis.
Shabaninejad H, Kenny RP, Robinson T, Stoniute A, O'Keefe H, Still M, Thornton C, Pearson F, Beyer F, Meader N. Shabaninejad H, et al. Health Technol Assess. 2024 Oct;28(75):1-75. doi: 10.3310/TGAC4201. Health Technol Assess. 2024. PMID: 39487741 Free PMC article. - Undernutrition as a risk factor for tuberculosis disease.
Franco JV, Bongaerts B, Metzendorf MI, Risso A, Guo Y, Peña Silva L, Boeckmann M, Schlesinger S, Damen JA, Richter B, Baddeley A, Bastard M, Carlqvist A, Garcia-Casal MN, Hemmingsen B, Mavhunga F, Manne-Goehler J, Viney K. Franco JV, et al. Cochrane Database Syst Rev. 2024 Jun 11;6(6):CD015890. doi: 10.1002/14651858.CD015890.pub2. Cochrane Database Syst Rev. 2024. PMID: 38860538 Free PMC article. Review.
Cited by
- The monogenic basis of human tuberculosis.
Boisson-Dupuis S. Boisson-Dupuis S. Hum Genet. 2020 Jun;139(6-7):1001-1009. doi: 10.1007/s00439-020-02126-6. Epub 2020 Feb 13. Hum Genet. 2020. PMID: 32055999 Free PMC article. Review. - Laboratory evaluation of the IFN-γ circuit for the molecular diagnosis of Mendelian susceptibility to mycobacterial disease.
Esteve-Solé A, Sologuren I, Martínez-Saavedra MT, Deyà-Martínez À, Oleaga-Quintas C, Martinez-Barricarte R, Martin-Nalda A, Juan M, Casanova JL, Rodriguez-Gallego C, Alsina L, Bustamante J. Esteve-Solé A, et al. Crit Rev Clin Lab Sci. 2018 May;55(3):184-204. doi: 10.1080/10408363.2018.1444580. Epub 2018 Mar 4. Crit Rev Clin Lab Sci. 2018. PMID: 29502462 Free PMC article. Review. - Inherited human IFN-γ deficiency underlies mycobacterial disease.
Kerner G, Rosain J, Guérin A, Al-Khabaz A, Oleaga-Quintas C, Rapaport F, Massaad MJ, Ding JY, Khan T, Ali FA, Rahman M, Deswarte C, Martinez-Barricarte R, Geha RS, Jeanne-Julien V, Garcia D, Chi CY, Yang R, Roynard M, Fleckenstein B, Rozenberg F, Boisson-Dupuis S, Ku CL, Seeleuthner Y, Béziat V, Marr N, Abel L, Al-Herz W, Casanova JL, Bustamante J. Kerner G, et al. J Clin Invest. 2020 Jun 1;130(6):3158-3171. doi: 10.1172/JCI135460. J Clin Invest. 2020. PMID: 32163377 Free PMC article. - A purely quantitative form of partial recessive IFN-γR2 deficiency caused by mutations of the initiation or second codon.
Oleaga-Quintas C, Deswarte C, Moncada-Vélez M, Metin A, Krishna Rao I, Kanık-Yüksek S, Nieto-Patlán A, Guérin A, Gülhan B, Murthy S, Özkaya-Parlakay A, Abel L, Martínez-Barricarte R, Pérez de Diego R, Boisson-Dupuis S, Kong XF, Casanova JL, Bustamante J. Oleaga-Quintas C, et al. Hum Mol Genet. 2018 Nov 15;27(22):3919-3935. doi: 10.1093/hmg/ddy275. Hum Mol Genet. 2018. PMID: 31222290 Free PMC article. - An Updated Review on MSMD Research Globally and A Literature Review on the Molecular Findings, Clinical Manifestations, and Treatment Approaches in China.
Xia L, Liu XH, Yuan Y, Lowrie DB, Fan XY, Li T, Hu ZD, Lu SH. Xia L, et al. Front Immunol. 2022 Jul 18;13:926781. doi: 10.3389/fimmu.2022.926781. eCollection 2022. Front Immunol. 2022. PMID: 36569938 Free PMC article. Review.
References
- Casanova JL, Abel L. Genetic dissection of immunity to mycobacteria: the human model. Annu Rev Immunol. 2002;20:581–620. - PubMed
- Schepers K, Schandené L, Bustamante J, Vooren JP, Suremain M, Casanova JL, Yombi J, Jacobs F, Mascart F, Goffard JC. IL-12Rβ1 Deficiency and Disseminated Mycobacterium tilburgii Disease. J Clin Immunol. 2013;33:1285–8. - PubMed
- de Beaucoudrey L, Samarina A, Bustamante J, Cobat A, Boisson-Dupuis S, Feinberg J, Al-Muhsen S, Jannière L, Rose Y, de Suremain M, Kong XF, Filipe-Santos O, Chapgier A, Picard C, Fischer A, Dogu F, Ikinciogullari A, Tanir G, Al-Hajjar S, Al-Jumaah S, Frayha HH, AlSum Z, Al-Ajaji S, Alangari A, Al-Ghonaium A, Adimi P, Mansouri D, Ben-Mustapha I, Yancoski J, Garty BZ, Rodriguez-Gallego C, Caragol I, Kutukculer N, Kumararatne DS, Patel S, Doffinger R, Exley A, Jeppsson O, Reichenbach J, Nadal D, Boyko Y, Pietrucha B, Anderson S, Levin M, Schandené L, Schepers K, Efira A, Mascart F, Matsuoka M, Sakai T, Siegrist CA, Frecerova K, Blüetters-Sawatzki R, Bernhöft J, Freihorst J, Baumann U, Richter D, Haerynck F, De Baets F, Novelli V, Lammas D, Vermylen C, Tuerlinckx D, Nieuwhof C, Pac M, Haas WH, Müller-Fleckenstein I, Fleckenstein B, Levy J, Raj R, Cohen AC, Lewis DB, Holland SM, Yang KD, Wang X, Wang X, Jiang L, Yang X, Zhu C, Xie Y, Lee PPW, Chan KW, Chen TX, Castro G, Natera I, Codoceo A, King A, Bezrodnik L, Di Giovani D, Gaillard MI, de Moraes-Vasconcelos D, Grumach AS, da Silva Duarte AJ, Aldana R, Espinosa-Rosales FJ, Bejaoui M, Bousfiha AA, Baghdadi JE, Özbek N, Aksu G, Keser M, Somer A, Hatipoglu N, Aydogmus Ç, Asilsoy S, Camcioglu Y, Gülle S, Ozgur TT, Ozen M, Oleastro M, Bernasconi A, Mamishi S, Parvaneh N, Rosenzweig S, Barbouche R, Pedraza S, Lau YL, Ehlayel MS, Fieschi C, Abel L, Sanal O, Casanova JL. Revisiting human IL-12Rβ1 deficiency: a survey of 141 patients from 30 countries. Medicine. 2010;89:381–402. doi: 10.1097/MD.0b013e3181fdd832. - DOI - PMC - PubMed
- Filipe-Santos O, Bustamante J, Chapgier A, Vogt G, de Beaucoudrey L, Feinberg J, Jouanguy E, Boisson-Dupuis S, Fieschi C, Picard C, Casanova JL. Inborn errors of IL-12/23- and IFN-γ-mediated immunity: molecular, cellular, and clinical features. Semin Immunol. 2006;18:347–61. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R37 AI095983/AI/NIAID NIH HHS/United States
- UL1 TR000043/TR/NCATS NIH HHS/United States
- 5R37AI095983/AI/NIAID NIH HHS/United States
- 8UL1TR000043/TR/NCATS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical