Narcolepsy patients have antibodies that stain distinct cell populations in rat brain and influence sleep patterns - PubMed (original) (raw)
. 2014 Sep 2;111(35):E3735-44.
doi: 10.1073/pnas.1412189111. Epub 2014 Aug 18.
Csaba Adori 2, Szilvia Vas 3, Ylva Kai-Larsen 4, Tomi Sarkanen 5, Andreas Cederlund 6, Birgitta Agerberth 7, Ilkka Julkunen 8, Beata Horvath 9, Diana Kostyalik 9, Lajos Kalmár 10, Gyorgy Bagdy 3, Anne Huutoniemi 11, Markku Partinen 12, Tomas Hökfelt 2
Affiliations
- PMID: 25136085
- PMCID: PMC4156690
- DOI: 10.1073/pnas.1412189111
Narcolepsy patients have antibodies that stain distinct cell populations in rat brain and influence sleep patterns
Peter Bergman et al. Proc Natl Acad Sci U S A. 2014.
Abstract
Narcolepsy is a chronic sleep disorder, likely with an autoimmune component. During 2009 and 2010, a link between A(H1N1)pdm09 Pandemrix vaccination and onset of narcolepsy was suggested in Scandinavia. In this study, we searched for autoantibodies related to narcolepsy using a neuroanatomical array: rat brain sections were processed for immunohistochemistry/double labeling using patient sera/cerebrospinal fluid as primary antibodies. Sera from 89 narcoleptic patients, 52 patients with other sleep-related disorders (OSRDs), and 137 healthy controls were examined. Three distinct patterns of immunoreactivity were of particular interest: pattern A, hypothalamic melanin-concentrating hormone and proopiomelanocortin but not hypocretin/orexin neurons; pattern B, GABAergic cortical interneurons; and pattern C, mainly globus pallidus neurons. Altogether, 24 of 89 (27%) narcoleptics exhibited pattern A or B or C. None of the patterns were exclusive for narcolepsy but were also detected in the OSRD group at significantly lower numbers. Also, some healthy controls exhibited these patterns. The antigen of pattern A autoantibodies was identified as the common C-terminal epitope of neuropeptide glutamic acid-isoleucine/α-melanocyte-stimulating hormone (NEI/αMSH) peptides. Passive transfer experiments on rat showed significant effects of pattern A human IgGs on rapid eye movement and slow-wave sleep time parameters in the inactive phase and EEG θ-power in the active phase. We suggest that NEI/αMSH autoantibodies may interfere with the fine regulation of sleep, contributing to the complex pathogenesis of narcolepsy and OSRDs. Also, patterns B and C are potentially interesting, because recent data suggest a relevance of those brain regions/neuron populations in the regulation of sleep/arousal.
Keywords: H1N1 vaccination; POMC neurons; autoantigen; neurotransmitter.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
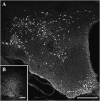
Fig. 1.
Demonstration of the NEI/αMSH staining pattern. (A) Low-power overview from a glycosidase predigested section of a colchicine-treated rat brain incubated with group A serum: a distinct neuron population is present in the ZI-LH region and Arc. (B) A dense fiber network but no cell bodies are seen in rats not treated with colchicine. (Scale bars: A, 500 µm; B, 200 µm.)
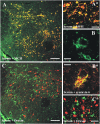
Fig. 2.
Characterization of the NEI/αMSH staining pattern in the ZI-LH region (group A). (A and A*) Fluorescent cells are identified as the MCH+ population. (C and C*) These cells are never immunoreactive for Hcrt/Orx. (B) High-power magnification reveals a dot-like subcellular pattern (100× objective, 1.5 digital zoom; merge of six _Z_-stack 0.5-µm-thick optical-layer micrographs). (B*) Serum staining was distinctly colocalized with syntaxin-6, a Golgi apparatus marker. Green channel, serum staining; red channel, MCH/Hcrt/syntaxin-6. (Scale bars: A and C, 200 µm; B and B*, 10 µm; A* and C*, 30 µm.)
Fig. 3.
Characterization of the NEI/αMSH staining pattern in the Arc (group A) and the cortical interneuron staining pattern (group B). (A–C) Serum+ cells are identical to the POMC-immunoreactive Arc neurons. (D) Only a few serum+ cortical fibers are double stained for MCH (insular cortex). The dense serum+ fiber plexus in the (E) paraventricular thalamic nucleus and (F) ventrolateral/lateral periaqueductal gray strongly colocalizes β-endorphin. (G–I) All pattern B serum+ interneurons express GAD67-EGFP; (J–L) most but not all pattern B interneurons (parietal cortex) are positive for (J) calbindin but never (L) calretinin, and (K) a few are somatostatin+. (M–O) In one group B case (insular cortex), however, nearly all serum+ interneurons are (O) calretinin+ and (N) occasionally, vasoactive intestinal polypeptide+ (VIP+) but never (M) calbindin+. Green channel: (A, C, D–F, and J–O) serum, (H and I) GAD67-EGFP; red channel: (G and I) serum, (B, C, E, and F) β-endorphin, (D) MCH, (J and M) calbindin, (K) somatostatin, (L and O) calretinin, or (N) VIP. (Scale bars: 100 μm.)
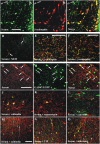
Fig. 4.
Characterization of patterns B and C neurons. Cortical bi- and multipolar interneurons are seen in (A) the hippocampus and (B) parietal cortex after incubation with group B serum, and (C) in the parietal cortex after incubation with group B CSF. Multipolar neurons with dendritic processes and axons are stained in the (D) globus pallidus and (E) piriform cortex after incubation with group C serum. (Scale bars: A–C and E, 100 µm; D, 200 µm.)
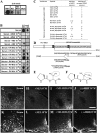
Fig. 5.
Characterization of the immunogenic peptides. Pattern A IgGs recognize a common epitope in the C terminus of αMSH and NEI. (A) Pattern A serum (1:5,000) recognizes αMSH- and NEI-matured peptides (0.6 nmol) and less strongly, NEI-MCH peptide (0.6 nmol; preproMCH [131–165]) but not the matured MCH peptide in the dot blot test. (B) Preincubation of pattern A serum (1:5,000) with 10−5 or 10−6 M αMSH or NEI peptides distinctly attenuates or completely abolishes the binding of serum to NEI or αMSH peptide (0.6 nmol). Preincubation of the same pattern A serum with 10−6–10−3M PV-NH2 or PI-NH2 dipeptides concentration-dependently decreases the binding of serum to NEI or αMSH peptide (0.6 nmol). (C) Summary of adsorption experiments: +, staining intensity does not change; ↓, staining intensity is decreased; ↓↓, staining intensity is highly decreased; 0, staining is completely abolished. (D) Schematic presentation of proMCH and the enzymatic processes leading to formation of matured NEI and MCH peptide. Prohormone-convertase (PC) first cleaves on the C-terminal side of the KR and RR sequences. Carboxypeptidase-C (CpE) removes the RR dibasic extensions. Then, peptidylglycine-α-amidating-monooxygenase (PAM) generates an amide group of G and the C terminus of I in the NEI peptide. (Amidated NEI peptide is the active form in the brain.) Note that the C terminus of αMSH peptide contains an amidated PV C-terminal motif (double underlined). *Acetylation site on the αMSH peptide. Modified from ref. . (E and F) Note the high structural similarities between (E) PV-NH2 and (F) PI-NH2 dipeptides (the difference is only a methylene group; circled). (G–N) Representative micrographs of adsorption experiments showing (G–J) the perifornical region (MCH neurons) and (K–N) Arc (POMC neurons). Note that NEI and αMSH peptides at 10−5 M completely abolish the immunostaining, whereas NEI-MCH peptide at 10−4 M concentration distinctly decreases the staining. (Scale bars: G–J, 200 µm; K–N, 100 µm.)
Fig. 6.
(A–I) Physiological effects of pattern A IgG on the sleep architecture. Graphical representation of different vigilance stages and sleep fragmentation [first 6 h, inactive (light) phase of D2] after icv. injection of IgG preparations (65 mg/mL) from (A, D, and G) an HC case (HC-IgG), (B, E, and H) a narcoleptic case with no staining pattern (NP-IgG), and (C, F, and I) a narcoleptic case with an staining pattern (NEI/αMSH-IgG) compared with the BL recordings. Sleep fragmentation is increased in the cases of both narcoleptic IgG preparations (B and C vs. A). The SWS2/SWS1 ratio and the time spent in REM are selectively decreased only in the case of the NEI/αMSH-IgG (F vs. D and E and I vs. G and H; SWS2/SWS1 and time spent in REM, respectively). Statistical analysis: two-way repeated measure ANOVA matched by pairs (repeated factor: hours 1–6); each group was compared with its own BL. *P < 0.05, Bonferroni multiple comparisons posthoc test for every 1 h. (J–L) Graphical representation of the number of REM episodes during the first 6 h of the inactive phase (D2 and D15). The number of REM episodes significantly decreased only in the NEI/αMSH-IgG group at both time points (L vs. J and K). Statistical analysis: one-way repeated measure ANOVA. *P < 0.05, Tukey posthoc test. (M–O) Graphical representation of the numbers of NREM → wake and NREM → REM transitions (first 6 h, inactive phase, and D2) after the injection (N and O vs. M). The number of NREM → wake transitions increased with both narcoleptic sera, whereas the number of NREM → REM decreased selectively with the NEI/αMSH pattern IgG (O vs. M and N). Statistical analysis: one-way repeated measure ANOVA. *P < 0.05, Tukey posthoc test. (P) Summary of the statistically significant alterations in sleep architecture compared with BL recordings. *Significant results of two-way repeated measure ANOVA or one-way repeated measure ANOVA. In the HC and NP groups, no significant effects were observed at D15 (not shown). n.s., not significant. (Q–S) Representative micrographs of the LH (colchicine-treated rat) stained with (Q) serum, (R) CSF, or (S) IgG preparation from the same group A patient all showing the same NEI/αMSH. (Scale bar: Q–S, 200 µm.)
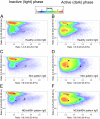
Fig. 7.
Effect of icv.-injected IgG from (A and B) an HC, from (C and D) an NC without the NEI/αMSH pattern, and (E and F) an NC with the NEI/αMSH pattern on the distribution and density of EEG power on a 2D-state space heat map in passive and active phases. Plotting the spectral ratios of EEG power data (ratio 1 on the x axis and ratio 2 on the y axis) separated three distinct clusters of EEG power points: right, REM sleep; upper left, NREM sleep; lower left, wake. Each plot represents EEG power data (including all animals per groups) of 6-h recordings on color-coded density maps (digits on the scale show the number of overlapping epochs on a given area). Centroids of different sleep stages are indicated by circles (black, BL; white, day 2). Noteworthy, REM point of NEI/αMSH group shifted toward wake in the active (dark) phase on day 2 vs. BL. The method is modified from ref. .
Similar articles
- Neuronal Antibodies in Children with or without Narcolepsy following H1N1-AS03 Vaccination.
Thebault S, Waters P, Snape MD, Cottrell D, Darin N, Hallböök T, Huutoniemi A, Partinen M, Pollard AJ, Vincent A. Thebault S, et al. PLoS One. 2015 Jun 19;10(6):e0129555. doi: 10.1371/journal.pone.0129555. eCollection 2015. PLoS One. 2015. PMID: 26090827 Free PMC article. - Autoantibodies in Pandemrix®-induced narcolepsy: Nine candidate autoantigens fail the conformational autoantibody test.
Wallenius M, Lind A, Akel O, Karlsson E, Svensson M, Arvidsson E, Ramelius A, Törn C, Palm L, Lernmark Å, Elding Larsson H. Wallenius M, et al. Autoimmunity. 2019 Jun;52(4):185-191. doi: 10.1080/08916934.2019.1643843. Epub 2019 Jul 22. Autoimmunity. 2019. PMID: 31328572 - A/H1N1 antibodies and TRIB2 autoantibodies in narcolepsy patients diagnosed in conjunction with the Pandemrix vaccination campaign in Sweden 2009-2010.
Lind A, Ramelius A, Olsson T, Arnheim-Dahlström L, Lamb F, Khademi M, Ambati A, Maeurer M, Nilsson AL, Bomfim IL, Fink K, Lernmark Å. Lind A, et al. J Autoimmun. 2014 May;50:99-106. doi: 10.1016/j.jaut.2014.01.031. Epub 2014 Jan 29. J Autoimmun. 2014. PMID: 24485154 - Narcolepsy and the hypocretins.
Wurtman RJ. Wurtman RJ. Metabolism. 2006 Oct;55(10 Suppl 2):S36-9. doi: 10.1016/j.metabol.2006.07.011. Metabolism. 2006. PMID: 16979425 Review. - Narcolepsy in Parkinson's disease.
Haq IZ, Naidu Y, Reddy P, Chaudhuri KR. Haq IZ, et al. Expert Rev Neurother. 2010 Jun;10(6):879-84. doi: 10.1586/ern.10.56. Expert Rev Neurother. 2010. PMID: 20518604 Review.
Cited by
- Association of Rare Immune-Related Adverse Events to Survival in Advanced Cancer Patients Treated with Immune Checkpoint Inhibitors: A Real-World Single-Center Cohort Study.
Kuusisalo S, Koivunen JP, Iivanainen S. Kuusisalo S, et al. Cancers (Basel). 2022 May 3;14(9):2276. doi: 10.3390/cancers14092276. Cancers (Basel). 2022. PMID: 35565405 Free PMC article. - Autoantibodies reactive to adrenocorticotropic hormone can alter cortisol secretion in both aggressive and nonaggressive humans.
Værøy H, Adori C, Legrand R, Lucas N, Breton J, Cottard C, do Rego JC, Duparc C, Louiset E, Lefebvre H, Déchelotte P, Western E, Andersson S, Hökfelt T, Fetissov SO. Værøy H, et al. Proc Natl Acad Sci U S A. 2018 Jul 10;115(28):E6576-E6584. doi: 10.1073/pnas.1720008115. Epub 2018 Jun 25. Proc Natl Acad Sci U S A. 2018. PMID: 29941562 Free PMC article. Clinical Trial. - Absence of specific autoantibodies in patients with narcolepsy type 1 as indicated by an unbiased random peptide-displayed phage screening.
Tran TT, Nguyen TN, Dauvilliers Y, Liblau R, Nguyen XH. Tran TT, et al. PLoS One. 2024 Mar 5;19(3):e0297625. doi: 10.1371/journal.pone.0297625. eCollection 2024. PLoS One. 2024. PMID: 38442093 Free PMC article. - CD8 T cell-mediated killing of orexinergic neurons induces a narcolepsy-like phenotype in mice.
Bernard-Valnet R, Yshii L, Quériault C, Nguyen XH, Arthaud S, Rodrigues M, Canivet A, Morel AL, Matthys A, Bauer J, Pignolet B, Dauvilliers Y, Peyron C, Liblau RS. Bernard-Valnet R, et al. Proc Natl Acad Sci U S A. 2016 Sep 27;113(39):10956-61. doi: 10.1073/pnas.1603325113. Epub 2016 Sep 12. Proc Natl Acad Sci U S A. 2016. PMID: 27621438 Free PMC article. - Orexins as Novel Therapeutic Targets in Inflammatory and Neurodegenerative Diseases.
Couvineau A, Voisin T, Nicole P, Gratio V, Abad C, Tan YV. Couvineau A, et al. Front Endocrinol (Lausanne). 2019 Oct 22;10:709. doi: 10.3389/fendo.2019.00709. eCollection 2019. Front Endocrinol (Lausanne). 2019. PMID: 31695678 Free PMC article. Review.
References
- Dauvilliers Y, Arnulf I, Mignot E. Narcolepsy with cataplexy. Lancet. 2007;369(9560):499–511. - PubMed
- Longstreth WT, Jr, Koepsell TD, Ton TG, Hendrickson AF, van Belle G. The epidemiology of narcolepsy. Sleep. 2007;30(1):13–26. - PubMed
- David A, Constantino F, dos Santos JM, Paiva T. Health-related quality of life in Portuguese patients with narcolepsy. Sleep Med. 2012;13(3):273–277. - PubMed
- Sakurai T, et al. Orexins and orexin receptors: A family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92(4):573–585. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous