Somatic mutations in cerebral cortical malformations - PubMed (original) (raw)
. 2014 Aug 21;371(8):733-43.
doi: 10.1056/NEJMoa1314432.
Anh-Thu N Lam, Martin Kircher, Alissa M D'Gama, Jian Wang, Brenda J Barry, Xiaochang Zhang, Robert Sean Hill, Jennifer N Partlow, Aldo Rozzo, Sarah Servattalab, Bhaven K Mehta, Meral Topcu, Dina Amrom, Eva Andermann, Bernard Dan, Elena Parrini, Renzo Guerrini, Ingrid E Scheffer, Samuel F Berkovic, Richard J Leventer, Yiping Shen, Bai Lin Wu, A James Barkovich, Mustafa Sahin, Bernard S Chang, Michael Bamshad, Deborah A Nickerson, Jay Shendure, Annapurna Poduri, Timothy W Yu, Christopher A Walsh
Affiliations
- PMID: 25140959
- PMCID: PMC4274952
- DOI: 10.1056/NEJMoa1314432
Somatic mutations in cerebral cortical malformations
Saumya S Jamuar et al. N Engl J Med. 2014.
Abstract
Background: Although there is increasing recognition of the role of somatic mutations in genetic disorders, the prevalence of somatic mutations in neurodevelopmental disease and the optimal techniques to detect somatic mosaicism have not been systematically evaluated.
Methods: Using a customized panel of known and candidate genes associated with brain malformations, we applied targeted high-coverage sequencing (depth, ≥200×) to leukocyte-derived DNA samples from 158 persons with brain malformations, including the double-cortex syndrome (subcortical band heterotopia, 30 persons), polymicrogyria with megalencephaly (20), periventricular nodular heterotopia (61), and pachygyria (47). We validated candidate mutations with the use of Sanger sequencing and, for variants present at unequal read depths, subcloning followed by colony sequencing.
Results: Validated, causal mutations were found in 27 persons (17%; range, 10 to 30% for each phenotype). Mutations were somatic in 8 of the 27 (30%), predominantly in persons with the double-cortex syndrome (in whom we found mutations in DCX and LIS1), persons with periventricular nodular heterotopia (FLNA), and persons with pachygyria (TUBB2B). Of the somatic mutations we detected, 5 (63%) were undetectable with the use of traditional Sanger sequencing but were validated through subcloning and subsequent sequencing of the subcloned DNA. We found potentially causal mutations in the candidate genes DYNC1H1, KIF5C, and other kinesin genes in persons with pachygyria.
Conclusions: Targeted sequencing was found to be useful for detecting somatic mutations in patients with brain malformations. High-coverage sequencing panels provide an important complement to whole-exome and whole-genome sequencing in the evaluation of somatic mutations in neuropsychiatric disease. (Funded by the National Institute of Neurological Disorders and Stroke and others.).
Figures
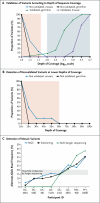
Figure 1. Specificity Analysis of the Deep Targeted-Sequencing Technique
Panel A shows the validation rate versus the depth of sequence coverage for germline and mosaic variants that were and were not validated successfully. Variants detected at a depth of more than 100× (blue shaded area) were more likely to be validated. Panel B is an enlarged view of the pink shaded and unshaded areas in Panel A. Variants detected at a depth of less than 60× (pink shaded area) were less likely to be validated than those detected at a depth of greater than 60×. This was especially true of mosaic variants. Panel C shows the proportion of mosaic variants detected by next-generation sequencing (NGS), which was similar to that detected by subcloning. When Sanger sequenc- ing was used, the mosaic variants at allele frequencies below 17% (in Participants DC-4601, DC-4401, DC- 401, and DC-5103, all of whom had the double-cortex syndrome) were not detected, and mutations present at high allele frequencies (at approximately 35% in Participant PH-16001, who had periventricular nodular heterotopia, and approximately 23% in Participant PAC-902, who had pachygyria) resembled germline mutations. The mosaic variant in Participant DC-2101 was missed in the initial analysis and was detected as a small peak only on directed examination of the Sanger chromatogram.
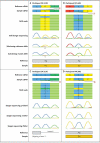
Figure 2. Detection of Variants by Means of NGS and Sanger Sequencing
The figure shows NGS reads aligned with Sanger se- quencing chromatograms from bulk DNA amplifica- tion and after subcloning for Participant DC-5103 (Panel A) and Participant DC-4401 (Panel B); the vari- ants (circled nucleotides) were detected in a fraction of the reads but were missed when targeted Sanger sequencing was used. Subcloning detected the vari- ants in a proportion of clones, and the proportion of NGS reads was strongly correlated with the propor- tion of clones containing a mosaic read. NGS reads aligned with Sanger sequencing chromatograms from bulk DNA amplification of samples from Participant DC-2101 (Panel C) and Participant PAC-902 (Panel D) and their respective parents show that the mosaic variant (circled nucleotide) was detected as a smaller peak, as compared with the reference allele, on Sanger sequencing and arose de novo (i.e., it was absent from the parents). For the splicing mutation in Participant DC-2101, the prediction software did not enable a choice from among the potential splice-acceptor sites; therefore, no amino acid is depicted.
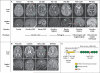
Figure 3. MRI Features of Brain Malformations Associated with Germline and Mosaic Variants, and Positions of Variants within the DYNC1H1 Protein
Panel A shows axial and midline sagittal MRI scans of the head of a person without neurologic abnormalities; two persons with the double-cortex syndrome (white arrows), associated with germline variants in one (Participant DC-7502) and a mosaic variant in the other (Participant DC-4601); two participants with pachygyria (white arrows), associated with germline variants in one (Participant PAC-1101) and a mosaic variant in the other (Participant PAC-902); a person with polymicrogyria (white arrows) and megalencephaly associated with a germline variant (Participant PMG-14201); and a person with periventricular nodular heterotopia (white arrows) associated with germline variants (Participant PH-4802). In addition, the sagittal image in Participant PAC-902 shows dysgenesis of the corpus callo- sum (arrowhead) and hypoplastic cerebellar vermis (white arrow), and the sagittal image of Participant 14201 shows cerebellar tonsillar herniation (white arrow) due to mass effect from the enlarged cerebral hemispheres. In Panel B, axial and midline sagittal MRI scans in the four persons with de novo mutations in DYNC1H1 show posterior-predominant pachygyria (white arrows in axial views in Partici- pants PAC-1601 and LIS-8201), thick, irregular cortex in the perisylvian region (white arrows in scans from Participants PMG-17401 and BFP-601), and a mildly dysmorphic corpus callosum (arrowheads in sagittal views in Participants LIS-8201, PMG-1704, and BFP-601). Panel C is a schematic representation of the DYNC1H1 protein, showing the positions of the alterations (black arrows) detected in our study.
Comment in
- Somatic mutations in cerebral cortical malformations.
Jamuar SS, Walsh CA. Jamuar SS, et al. N Engl J Med. 2014 Nov 20;371(21):2038. doi: 10.1056/NEJMc1411784. N Engl J Med. 2014. PMID: 25409382 No abstract available. - Somatic mutations in cerebral cortical malformations.
Morita H, Komuro I. Morita H, et al. N Engl J Med. 2014 Nov 20;371(21):2037. doi: 10.1056/NEJMc1411784. N Engl J Med. 2014. PMID: 25409383 No abstract available.
Similar articles
- [Epileptogenic brain malformations: radiological and clinical presentation and indications for genetic testing].
Bahi-Buisson N, Boddaert N, Saillour Y, Souville I, Poirier K, Léger PL, Castelnau L, Plouin P, Carion N, Beldjord C, Chelly J. Bahi-Buisson N, et al. Rev Neurol (Paris). 2008 Dec;164(12):995-1009. doi: 10.1016/j.neurol.2008.04.006. Epub 2008 Jun 9. Rev Neurol (Paris). 2008. PMID: 18808783 Review. French. - Identification of DCX gene mutation in lissencephaly spectrum with subcortical band heterotopia using whole exome sequencing.
Jang MA, Woo HI, Kim JW, Lee J, Ki CS. Jang MA, et al. Pediatr Neurol. 2013 May;48(5):411-4. doi: 10.1016/j.pediatrneurol.2012.12.033. Pediatr Neurol. 2013. PMID: 23583063 - Analysis of 17 genes detects mutations in 81% of 811 patients with lissencephaly.
Di Donato N, Timms AE, Aldinger KA, Mirzaa GM, Bennett JT, Collins S, Olds C, Mei D, Chiari S, Carvill G, Myers CT, Rivière JB, Zaki MS; University of Washington Center for Mendelian Genomics; Gleeson JG, Rump A, Conti V, Parrini E, Ross ME, Ledbetter DH, Guerrini R, Dobyns WB. Di Donato N, et al. Genet Med. 2018 Nov;20(11):1354-1364. doi: 10.1038/gim.2018.8. Epub 2018 Apr 19. Genet Med. 2018. PMID: 29671837 Free PMC article. - Targeted re-sequencing in malformations of cortical development: genotype-phenotype correlations.
Accogli A, Severino M, Riva A, Madia F, Balagura G, Iacomino M, Carlini B, Baldassari S, Giacomini T, Croci C, Pisciotta L, Messana T, Boni A, Russo A, Bilo L, Tonziello R, Coppola A, Filla A, Mecarelli O, Casalone R, Pisani F, Falsaperla R, Marino S, Parisi P, Ferretti A, Elia M, Luchetti A, Milani D, Vanadia F, Silvestri L, Rebessi E, Parente E, Vatti G, Mancardi MM, Nobili L, Capra V, Salpietro V, Striano P, Zara F. Accogli A, et al. Seizure. 2020 Aug;80:145-152. doi: 10.1016/j.seizure.2020.05.023. Epub 2020 Jun 3. Seizure. 2020. PMID: 32570172 - Somatic mutation, genomic variation, and neurological disease.
Poduri A, Evrony GD, Cai X, Walsh CA. Poduri A, et al. Science. 2013 Jul 5;341(6141):1237758. doi: 10.1126/science.1237758. Science. 2013. PMID: 23828942 Free PMC article. Review.
Cited by
- Mutation in the mitochondrial chaperone TRAP1 leads to autism with more severe symptoms in males.
Rydzanicz M, Kuzniewska B, Magnowska M, Wójtowicz T, Stawikowska A, Hojka A, Borsuk E, Meyza K, Gewartowska O, Gruchota J, Miłek J, Wardaszka P, Chojnicka I, Kondrakiewicz L, Dymkowska D, Puścian A, Knapska E, Dziembowski A, Płoski R, Dziembowska M. Rydzanicz M, et al. EMBO Mol Med. 2024 Nov;16(11):2976-3004. doi: 10.1038/s44321-024-00147-6. Epub 2024 Sep 27. EMBO Mol Med. 2024. PMID: 39333440 Free PMC article. - PTPN11 and FLNA variants in a boy with ambiguous genitalia, short stature, and non-specific dysmorphic features.
Muranishi Y, Itonaga T, Ihara K, Katoh-Fukui Y, Tamaoka S, Hattori A, Kon M, Shinohara N, Fukami M. Muranishi Y, et al. Clin Pediatr Endocrinol. 2024;33(3):169-173. doi: 10.1297/cpe.2023-0074. Epub 2024 May 3. Clin Pediatr Endocrinol. 2024. PMID: 38993717 Free PMC article. - Congenital syndromic Chiari-like malformation (CSCM) in Holstein cattle: towards unravelling of possible genetic causes.
Jacinto JGP, Letko A, Häfliger IM, Drögemüller C, Agerholm JS. Jacinto JGP, et al. Acta Vet Scand. 2024 Jul 4;66(1):29. doi: 10.1186/s13028-024-00752-y. Acta Vet Scand. 2024. PMID: 38965607 Free PMC article. - Early Developmental Origins of Cortical Disorders Modeled in Human Neural Stem Cells.
Mato-Blanco X, Kim SK, Jourdon A, Ma S, Tebbenkamp ATN, Liu F, Duque A, Vaccarino FM, Sestan N, Colantuoni C, Rakic P, Santpere G, Micali N. Mato-Blanco X, et al. bioRxiv [Preprint]. 2024 Jun 14:2024.06.14.598925. doi: 10.1101/2024.06.14.598925. bioRxiv. 2024. PMID: 38915580 Free PMC article. Preprint. - Human embryonic genetic mosaicism and its effects on development and disease.
Waldvogel SM, Posey JE, Goodell MA. Waldvogel SM, et al. Nat Rev Genet. 2024 Oct;25(10):698-714. doi: 10.1038/s41576-024-00715-z. Epub 2024 Apr 11. Nat Rev Genet. 2024. PMID: 38605218 Review.
References
- Biesecker LG, Spinner NB. A genomic view of mosaicism and human disease. Nat Rev Genet. 2013;14:307–20. - PubMed
- Weinstein LS, Shenker A, Gejman PV, Merino MJ, Friedman E, Spiegel AM. Activating mutations of the stimulatory G protein in the McCune–Albright syndrome. N Engl J Med. 1991;325:1688–95. - PubMed
Publication types
MeSH terms
Grants and funding
- U54 HG006493/HG/NHGRI NIH HHS/United States
- RC2 MH089952/MH/NIMH NIH HHS/United States
- R01NS079277/NS/NINDS NIH HHS/United States
- 1RC2MH089952/MH/NIMH NIH HHS/United States
- R01 NS035129/NS/NINDS NIH HHS/United States
- K23NS069784/NS/NINDS NIH HHS/United States
- UM1 HG006493/HG/NHGRI NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- K23 NS069784/NS/NINDS NIH HHS/United States
- T32 GM007753/GM/NIGMS NIH HHS/United States
- T32GM07753/GM/NIGMS NIH HHS/United States
- HG006493/HG/NHGRI NIH HHS/United States
- R01NS035129/NS/NINDS NIH HHS/United States
- R01 NS079277/NS/NINDS NIH HHS/United States
- R01 NS073601/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous