Synthesis, properties, and biological activity of boranophosphate analogs of the mRNA cap: versatile tools for manipulation of therapeutically relevant cap-dependent processes - PubMed (original) (raw)
doi: 10.1093/nar/gku757. Epub 2014 Aug 22.
Anna Wypijewska del Nogal 1, Zbigniew M Darzynkiewicz 1, Janina Buck 2, Corina Nicola 2, Andreas N Kuhn 3, Maciej Lukaszewicz 1, Joanna Zuberek 1, Malwina Strenkowska 1, Marcin Ziemniak 1, Maciej Maciejczyk 4, Elzbieta Bojarska 5, Robert E Rhoads 6, Edward Darzynkiewicz 7, Ugur Sahin 3, Jacek Jemielity 8
Affiliations
- PMID: 25150148
- PMCID: PMC4176373
- DOI: 10.1093/nar/gku757
Synthesis, properties, and biological activity of boranophosphate analogs of the mRNA cap: versatile tools for manipulation of therapeutically relevant cap-dependent processes
Joanna Kowalska et al. Nucleic Acids Res. 2014.
Abstract
Modified mRNA cap analogs aid in the study of mRNA-related processes and may enable creation of novel therapeutic interventions. We report the synthesis and properties of 11 dinucleotide cap analogs bearing a single boranophosphate modification at either the α-, β- or γ-position of the 5',5'-triphosphate chain. The compounds can potentially serve either as inhibitors of translation in cancer cells or reagents for increasing expression of therapeutic proteins in vivo from exogenous mRNAs. The BH3-analogs were tested as substrates and binding partners for two major cytoplasmic cap-binding proteins, DcpS, a decapping pyrophosphatase, and eIF4E, a translation initiation factor. The susceptibility to DcpS was different between BH3-analogs and the corresponding analogs containing S instead of BH3 (S-analogs). Depending on its placement, the boranophosphate group weakened the interaction with DcpS but stabilized the interaction with eIF4E. The first of the properties makes the BH3-analogs more stable and the second, more potent as inhibitors of protein biosynthesis. Protein expression in dendritic cells was 2.2- and 1.7-fold higher for mRNAs capped with m2 (7,2'-O)GppBH3pG D1 and m2 (7,2'-O)GppBH3pG D2, respectively, than for in vitro transcribed mRNA capped with m2 (7,3'-O)GpppG. Higher expression of cancer antigens would make mRNAs containing m2 (7,2'-O)GppBH3pG D1 and m2 (7,2'-O)GppBH3pG D2 favorable for anticancer immunization.
© The Author(s) 2014. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures
Figure 1.
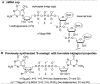
(A) Structure of the 5′-end of eukaryotic mRNA showing the cap and first three template nucleotide residues. (B) Structure of previously synthesized phosphorothioate cap analogs (S-analogs) that have favorable biological properties: m7GpSppG, a potent translational inhibitor, and m27,2′-_O_GppSpG, a reagent for enhancing the biological stability and translation efficiency of capped mRNAs.
Figure 2.
Structural comparison of phosphorothioate and boranophosphate moieties. (A) Electronic structures; (B) stereochemical structures. Both O to BH3 and O to S substitutions may result in P-diastereoisomerism. It should be noted, however, that the same spatial arrangement of substituents around stereogenic phosphorus center for phosphorothioate and boranophosphate groups produces different absolute configurations (SP and RP) because of the different priority of BH3 and S substituents according to Cahn–Ingold–Prelog priority rules. (C) A representative RP HPLC chromatogram of a mixture of two diastereomeric BH3-analogs (m7GppBH3pG D1 and D2; 2a and 2b). D1 denotes the isomer eluting faster from a reversed-phase (RP) HPLC column.
Figure 3.
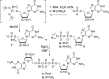
Synthesis of cap BH3-analogs 1 and 5 modified at the α-position of the triphosphate bridge. Abbreviations—BSA: _N,O_-bis(trimethylilyl)acetamide; ACN: acetonitrile.
Figure 4.
Synthetic routes for cap BH3-analog modified at the γ-position of the triphosphate bridge (3). (A) Attempted synthesis by coupling of 7-methylguanosine 5′-boranophosphate (10) and GDP imidazolide derivative. (B) A successful approach employing 7-methylguanosine 5′-(1-boranodiphosphate) (13) as the key intermediate.
Figure 5.
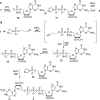
Synthesis of cap BH3-analogs 2 (A), 4 (B) and 6 (C) modified at the β-position of the triphosphate bridge.
Figure 6.
Synthesis of guanosine and adenosine α-boranotriphosphates and diadenosine 1,3-diboranotriphosphate by means of phosphorimidazolide chemistry.
Figure 7.
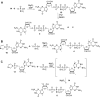
Representative HPLC profiles from the DcpS-susceptibility assay at lower enzyme concentration (Assay I). BH3-analogs were incubated at 40 μM with 100-nM human or C. elegans DcpS and aliquots taken at different time points were analyzed by RP HPLC at 260 nm as described in the Materials and Methods section_._ The analogs that were hydrolyzed in less than 10% within 2 h were assumed to be resistant to DcpS (see Supplementary Table S2). The black arrow in each panel indicates the retention time at which the reaction substrate is eluted. The initial degradation products of β-modified analogs are m7GMP and guanosine β-boranodiphosphate (GDPβBH3); however, the latter is chemically labile and rapidly hydrolyses to GMP during high-temperature deactivation of the enzyme (data not shown).
Figure 8.
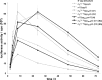
The influence of different mRNA cap analogs on luciferase expression in human immature dendritic cells (hiDCs). After electroporation of respective 5′-capped mRNAs into hiDCs, luciferase activity was measured after 2, 4, 8, 24, 48 and 72 h (each experiment was performed in duplicate). The corresponding averaged bioluminescence signals are depicted as a function of time. The data are shown as mean ± SD.
Figure 9.
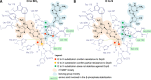
Effects of non-bridging phosphate chain modifications on the susceptibility of cap dinucleotides to cleavage by human DcpS: comparison between BH3-analogs (A) and S-analogs (B). The schematic map of plausible protein–ligand interactions is based on crystallographic structure of a catalytically inactivated hDcpS mutant (His 277→Asp) in complex with m7GpppG (PDB entry 1ST0 (17)). The main difference between the O to BH3 and O to S substitutions is that the β-BH3 substitutions produce DcpS-resistant analogs, whereas corresponding β-S-analogs are good substrates for DcpS. A possible explanation is that the β-boranophosphate moiety, due to insufficient ability of the BH3 substituent to form hydrogen bonds, cannot be sufficiently stabilized as a leaving group by the basic amino acid residues in DcpS's cap-binding pocket. Some ribose and nucleobase interactions have been omitted for clarity.
Similar articles
- Synthesis and characterization of mRNA cap analogs containing phosphorothioate substitutions that bind tightly to eIF4E and are resistant to the decapping pyrophosphatase DcpS.
Kowalska J, Lewdorowicz M, Zuberek J, Grudzien-Nogalska E, Bojarska E, Stepinski J, Rhoads RE, Darzynkiewicz E, Davis RE, Jemielity J. Kowalska J, et al. RNA. 2008 Jun;14(6):1119-31. doi: 10.1261/rna.990208. Epub 2008 Apr 22. RNA. 2008. PMID: 18430890 Free PMC article. - The first examples of mRNA cap analogs bearing boranophosphate modification.
Kowalska J, Zuberek J, Darzynkiewicz ZM, Lukaszewicz M, Darzynkiewicz E, Jemielity J. Kowalska J, et al. Nucleic Acids Symp Ser (Oxf). 2008;(52):289-90. doi: 10.1093/nass/nrn146. Nucleic Acids Symp Ser (Oxf). 2008. PMID: 18776367 - Phosphorothioate analogs of m7GTP are enzymatically stable inhibitors of cap-dependent translation.
Kowalska J, Lukaszewicz M, Zuberek J, Ziemniak M, Darzynkiewicz E, Jemielity J. Kowalska J, et al. Bioorg Med Chem Lett. 2009 Apr 1;19(7):1921-5. doi: 10.1016/j.bmcl.2009.02.053. Epub 2009 Feb 21. Bioorg Med Chem Lett. 2009. PMID: 19269171 - Synthesis of anti-reverse cap analogs (ARCAs) and their applications in mRNA translation and stability.
Grudzien-Nogalska E, Stepinski J, Jemielity J, Zuberek J, Stolarski R, Rhoads RE, Darzynkiewicz E. Grudzien-Nogalska E, et al. Methods Enzymol. 2007;431:203-27. doi: 10.1016/S0076-6879(07)31011-2. Methods Enzymol. 2007. PMID: 17923237 Review. - Potential therapeutic applications of RNA cap analogs.
Ziemniak M, Strenkowska M, Kowalska J, Jemielity J. Ziemniak M, et al. Future Med Chem. 2013 Jun;5(10):1141-72. doi: 10.4155/fmc.13.96. Future Med Chem. 2013. PMID: 23795970 Review.
Cited by
- Delivery of mRNA Therapeutics for the Treatment of Hepatic Diseases.
Trepotec Z, Lichtenegger E, Plank C, Aneja MK, Rudolph C. Trepotec Z, et al. Mol Ther. 2019 Apr 10;27(4):794-802. doi: 10.1016/j.ymthe.2018.12.012. Epub 2018 Dec 22. Mol Ther. 2019. PMID: 30655211 Free PMC article. Review. - Quantification of SARS-CoV-2 spike protein expression from mRNA vaccines using isotope dilution mass spectrometry.
Sutton WJH, Branham PJ, Williamson YM, Cooper HC, Najjar FN, Pierce-Ruiz CL, Barr JR, Williams TL. Sutton WJH, et al. Vaccine. 2023 Jun 13;41(26):3872-3884. doi: 10.1016/j.vaccine.2023.04.044. Epub 2023 May 8. Vaccine. 2023. PMID: 37202272 Free PMC article. - Opportunities and Challenges in the Delivery of mRNA-based Vaccines.
Wadhwa A, Aljabbari A, Lokras A, Foged C, Thakur A. Wadhwa A, et al. Pharmaceutics. 2020 Jan 28;12(2):102. doi: 10.3390/pharmaceutics12020102. Pharmaceutics. 2020. PMID: 32013049 Free PMC article. Review. - Self-Amplifying RNA Vaccines Give Equivalent Protection against Influenza to mRNA Vaccines but at Much Lower Doses.
Vogel AB, Lambert L, Kinnear E, Busse D, Erbar S, Reuter KC, Wicke L, Perkovic M, Beissert T, Haas H, Reece ST, Sahin U, Tregoning JS. Vogel AB, et al. Mol Ther. 2018 Feb 7;26(2):446-455. doi: 10.1016/j.ymthe.2017.11.017. Epub 2017 Dec 5. Mol Ther. 2018. PMID: 29275847 Free PMC article. - Recent Advances in Modified Cap Analogs: Synthesis, Biochemical Properties, and mRNA Based Vaccines.
Shanmugasundaram M, Senthilvelan A, Kore AR. Shanmugasundaram M, et al. Chem Rec. 2022 Aug;22(8):e202200005. doi: 10.1002/tcr.202200005. Epub 2022 Apr 14. Chem Rec. 2022. PMID: 35420257 Free PMC article. Review.
References
- Coller J., Parker R. Eukaryotic mRNA decapping. Annu. Rev. Biochem. 2004;73:861–890. - PubMed
- Sonenberg N. eIF4E, the mRNA cap-binding protein: from basic discovery to translational research. Biochem. Cell Biol. 2008;86:178–183. - PubMed
- Cougot N., van Dijk E., Babajko S., Séraphin B. ‘Cap-tabolism’. Trends Biochem. Sci. 2004;29:436–444. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous