Pharmacological inhibition of lipofuscin accumulation in the retina as a therapeutic strategy for dry AMD treatment - PubMed (original) (raw)
Pharmacological inhibition of lipofuscin accumulation in the retina as a therapeutic strategy for dry AMD treatment
Konstantin Petrukhin. Drug Discov Today Ther Strateg. 2013.
Abstract
Age-related macular degeneration (AMD) is the leading cause of blindness in the western world. There is no FDA-approved treatment for the most prevalent dry (atrophic) form of AMD. Photoreceptor degeneration in dry AMD is triggered by abnormalities in the retinal pigment epithelium (RPE). It has been suggested that excessive accumulation of fluorescent lipofuscin pigment in the RPE represents an important pathogenic factor in etiology and progression of dry AMD. Cytotoxic lipofuscin bisretinoids, such as A2E, are formed in the retina in a non-enzymatic way from visual cycle retinoids. Inhibition of toxic bisretinoid production in the retina seems to be a sound treatment strategy for dry AMD. In this review we discuss the following classes of pharmacological treatments inhibiting lipofuscin bisretinoid formation in the retina: direct inhibitors of key visual cycle enzymes, RBP4 antagonists, primary amine-containing aldehyde traps, and deuterated analogs of vitamin A.
Conflict of interest statement
Conflict of interest statement
Columbia University owns IP rights to ophthalmic use of A1120 and its derivatives
Figures
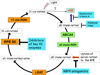
Figure 1. The visual cycle and points of intervention for pharmacological inhibition of lipofuscin bisretinoid synthesis in the retina
Enzymes and transporters facilitating visual cycle reactions in photoreceptor outer segments are highlighted in green. Enzymes catalyzing visual cycle reactions conducted in the RPE are shown in orange. Lipofuscin bisretinoid biosynthesis begins when all-trans-retinal leaves the visual cycle and non-enzymatically reacts with phosphatidylethanolamine forming the A2E precursor, A2-PE (blue arrow); 11-cis-retinal is also reported to be a precursor of A2E formation [30] (dotted arrow). Uptake of serum retinol to the RPE (red arrow) fuels the visual cycle. Highlighted in bright blue are four intervention points for suppression of lipofuscin bisretinoid formation: inhibition of key visual cycle enzymes such as RPE65, neutralization of free aldehydes, suppression of bisretinoid formation with deuterated vitamin A analogs, and reduction of retinol uptake to the retina with RBP4 antagonists. Abbreviations: ABCA4, retina-specific ABC transporter; all-trans-RDH, all-trans-retinol dehydrogenase; LRAT, lecithin-retinol acyltransferase; 11-cis-RDH, 11-cis-retinol-dehydrogenase.
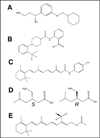
Figure 2. Structure of compounds exemplifying four classes of treatments capable of reducing lipofuscin bisretinoid production in animal models of enhanced retinal lipofuscinogenesis
A, Structure of the RPE65 inhibitor ACU-4429 as can be inferred from the US 2011/0003895-A1 patent application. A1120 (B) and fenretinide (C) exemplify the class of RBP4 antagonists. Racemic mixture of two primary amine-containing pegabalin stereoisomers (D) acts as an aldehyde trap. E, a deuterated C20-D3 vitamin A analog in a form of retinyl acetate.
Similar articles
- A non-retinoid antagonist of retinol-binding protein 4 rescues phenotype in a model of Stargardt disease without inhibiting the visual cycle.
Racz B, Varadi A, Kong J, Allikmets R, Pearson PG, Johnson G, Cioffi CL, Petrukhin K. Racz B, et al. J Biol Chem. 2018 Jul 20;293(29):11574-11588. doi: 10.1074/jbc.RA118.002062. Epub 2018 Jun 5. J Biol Chem. 2018. PMID: 29871924 Free PMC article. - Lipofuscin Granule Bisretinoid Oxidation in the Human Retinal Pigment Epithelium forms Cytotoxic Carbonyls.
Yakovleva M, Dontsov A, Trofimova N, Sakina N, Kononikhin A, Aybush A, Gulin A, Feldman T, Ostrovsky M. Yakovleva M, et al. Int J Mol Sci. 2021 Dec 25;23(1):222. doi: 10.3390/ijms23010222. Int J Mol Sci. 2021. PMID: 35008647 Free PMC article. - The bisretinoids of retinal pigment epithelium.
Sparrow JR, Gregory-Roberts E, Yamamoto K, Blonska A, Ghosh SK, Ueda K, Zhou J. Sparrow JR, et al. Prog Retin Eye Res. 2012 Mar;31(2):121-35. doi: 10.1016/j.preteyeres.2011.12.001. Epub 2011 Dec 22. Prog Retin Eye Res. 2012. PMID: 22209824 Free PMC article. Review. - Photodegradation of retinal bisretinoids in mouse models and implications for macular degeneration.
Ueda K, Zhao J, Kim HJ, Sparrow JR. Ueda K, et al. Proc Natl Acad Sci U S A. 2016 Jun 21;113(25):6904-9. doi: 10.1073/pnas.1524774113. Epub 2016 Jun 6. Proc Natl Acad Sci U S A. 2016. PMID: 27274068 Free PMC article. - Retinal ultrastructure of murine models of dry age-related macular degeneration (AMD).
Ramkumar HL, Zhang J, Chan CC. Ramkumar HL, et al. Prog Retin Eye Res. 2010 May;29(3):169-90. doi: 10.1016/j.preteyeres.2010.02.002. Epub 2010 Mar 3. Prog Retin Eye Res. 2010. PMID: 20206286 Free PMC article. Review.
Cited by
- RNA-seq analysis of ageing human retinal pigment epithelium: Unexpected up-regulation of visual cycle gene transcription.
Butler JM, Supharattanasitthi W, Yang YC, Paraoan L. Butler JM, et al. J Cell Mol Med. 2021 Jun;25(12):5572-5585. doi: 10.1111/jcmm.16569. Epub 2021 May 1. J Cell Mol Med. 2021. PMID: 33934486 Free PMC article. - Pgc-1α repression and high-fat diet induce age-related macular degeneration-like phenotypes in mice.
Zhang M, Chu Y, Mowery J, Konkel B, Galli S, Theos AC, Golestaneh N. Zhang M, et al. Dis Model Mech. 2018 Aug 16;11(9):dmm032698. doi: 10.1242/dmm.032698. Dis Model Mech. 2018. PMID: 29925537 Free PMC article. - Design, synthesis, and evaluation of nonretinoid retinol binding protein 4 antagonists for the potential treatment of atrophic age-related macular degeneration and Stargardt disease.
Cioffi CL, Dobri N, Freeman EE, Conlon MP, Chen P, Stafford DG, Schwarz DM, Golden KC, Zhu L, Kitchen DB, Barnes KD, Racz B, Qin Q, Michelotti E, Cywin CL, Martin WH, Pearson PG, Johnson G, Petrukhin K. Cioffi CL, et al. J Med Chem. 2014 Sep 25;57(18):7731-57. doi: 10.1021/jm5010013. Epub 2014 Sep 11. J Med Chem. 2014. PMID: 25210858 Free PMC article. - Visual cycle modulators versus placebo or observation for the prevention and treatment of geographic atrophy due to age-related macular degeneration.
Yeong JL, Loveman E, Colquitt JL, Royle P, Waugh N, Lois N. Yeong JL, et al. Cochrane Database Syst Rev. 2020 Dec 17;12(12):CD013154. doi: 10.1002/14651858.CD013154.pub2. Cochrane Database Syst Rev. 2020. PMID: 33331670 Free PMC article.
References
- Petrukhin K. New therapeutic targets in atrophic age-related macular degeneration. Expert Opin Ther Targets. 2007;11(5):625–639. - PubMed
- Young RW. Pathophysiology of age-related macular degeneration. Surv Ophthalmol. 1987;31(5):291–306. - PubMed
- Dorey CK, Wu G, Ebenstein D, Garsd A, Weiter JJ. Cell loss in the aging retina. Relationship to lipofuscin accumulation and macular degeneration. Invest Ophthalmol Vis Sci. 1989;30(8):1691–1699. - PubMed
- Holz FG, Bellmann C, Margaritidis M, Schutt F, Otto TP, Volcker HE. Patterns of increased in vivo fundus autofluorescence in the junctional zone of geographic atrophy of the retinal pigment epithelium associated with agerelated macular degeneration. Graefes Arch Clin Exp Ophthalmol. 1999;237(2):145–152. - PubMed
- Holz FG, Bindewald-Wittich A, Fleckenstein M, Dreyhaupt J, Scholl HP, Schmitz-Valckenberg S. Progression of geographic atrophy and impact of fundus autofluorescence patterns in age-related macular degeneration. Am J Ophthalmol. 2007;143(3):463–472. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous