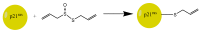Allicin: chemistry and biological properties - PubMed (original) (raw)
Review
Allicin: chemistry and biological properties
Jan Borlinghaus et al. Molecules. 2014.
Abstract
Allicin (diallylthiosulfinate) is a defence molecule from garlic (Allium sativum L.) with a broad range of biological activities. Allicin is produced upon tissue damage from the non-proteinogenic amino acid alliin (S-allylcysteine sulfoxide) in a reaction that is catalyzed by the enzyme alliinase. Current understanding of the allicin biosynthetic pathway will be presented in this review. Being a thiosulfinate, allicin is a reactive sulfur species (RSS) and undergoes a redox-reaction with thiol groups in glutathione and proteins that is thought to be essential for its biological activity. Allicin is physiologically active in microbial, plant and mammalian cells. In a dose-dependent manner allicin can inhibit the proliferation of both bacteria and fungi or kill cells outright, including antibiotic-resistant strains like methicillin-resistant Staphylococcus aureus (MRSA). Furthermore, in mammalian cell lines, including cancer cells, allicin induces cell-death and inhibits cell proliferation. In plants allicin inhibits seed germination and attenuates root-development. The majority of allicin's effects are believed to be mediated via redox-dependent mechanisms. In sub-lethal concentrations, allicin has a variety of health-promoting properties, for example cholesterol- and blood pressure-lowering effects that are advantageous for the cardio-vascular system. Clearly, allicin has wide-ranging and interesting applications in medicine and (green) agriculture, hence the detailed discussion of its enormous potential in this review. Taken together, allicin is a fascinating biologically active compound whose properties are a direct consequence of the molecule's chemistry.
Conflict of interest statement
The authors declare no conflict of interests.
Figures
Scheme 1
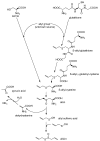
Biosynthesis of allicin: Based on Granroth’s work there are two possible biosynthetic pathways leading to S-allyl-cysteine. The detection of 14C-labeled S-allyl-cysteine after feeding plants with 14C-labeled serine and the incorporation of various alkyl mercaptans led Granroth to the conclusion that serine is one possible substrate for S-allyl-cysteine biosynthesis. An alternative pathway leads from glutathione to S-allyl-cysteine. This was confirmed by the detection of S-allyl-glutathione and S-allyl-γ-glutamyl-cysteine. The source of the allyl-group is still unknown. S-allyl-cysteine is oxidised to alliin, which is the “inactive” precursor of allicin. Alliin is enzymatically hydrolysed to produce allyl sulfenic acid which condenses spontaneously to allicin.
Scheme 2
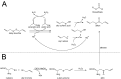
Synthesis of allicin according to Stoll and Seebeck: (A) Diallyl disulfide (distilled under reduced pressure) is mixed with acetic acid and hydrogen peroxide. Because hydrogen peroxide reacts very slowly with diallyl disulfide, acetic acid is needed as a catalyst. Peracetic acid (ethaneperoxoic acid) is formed, which is able to oxidize diallyl disulfide to allyl sulfenic acid. This reaction also leads to the production of allyl radicals which can react with hydrogen peroxide to form allyl sulfenic acid and hydroxyl radicals. The latter are able to react with diallyl disulfide to form allyl sulfenic acid and allyl radicals again. Two molecules of allyl sulfenic acid condense to allicin. This reaction mechanism is not only suitable to synthesize allicin but also other thiosulfinates. (B) To produce allicin by an enzymatic reaction alliin is needed. Cysteine is mixed with allyl bromide in an alkaline (NaOH) mixture of water and ethanol to obtain S-allyl cysteine. The latter can be oxidized with hydrogen peroxide to produce alliin. By an enzymatic reaction of alliin with alliinase, allicin is formed.
Scheme 3
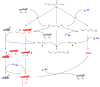
Overview of redox chemistry of allicin and cellular thiols: Allicin (1) is able to react with cellular thiols like glutathione (GSH) and cysteine-containing proteins. Reaction with proteins leads to S-allyl-mercapto-proteins (2) and allyl sulfenic acid (3). S-allyl-mercapto-proteins are able to react with other proteins by formation of disulfide bond-stabilised complexes (4) or to form intramolecular disulfide bonds (5). Both reactions lead to elimination of allyl mercaptan (6). Protein disulfide bonds can be reduced by cellular GSH which leads to S-glutathionyl-mercapto-proteins (7). To remove the glutathionyl residues from the proteins another GSH is needed. Allicin also reacts with GSH. This reaction leads to S-allyl-mercapto-glutathione (8) and allyl sulfenic acid (3). S-allyl-mercapto-glutathione can undergo a thiol/disulfide exchange reaction with another GSH to form GSSG and allyl mercaptan (6). Allyl sulfenic acid (3), produced in direct reactions of allicin and thiols is able to react with proteins to form S-allyl-mercapto-proteins (2), with GSH to form S-allyl-mercapto-glutathione (8), with allyl mercaptan (6) to DADS (9) or with another allyl sulfenic acid (3) to form allicin again.
Figure 1
Plate diffusion test. Antibacterial activity against E. coli DH5α demonstrated on seeded agar plates by different antibiotics is indicated by clear zones where growth inhibition has occurred. Freshly grown E. coli cells (OD600 = 0.2, 300 µL) were embedded in 20 mL warm agar in a Petri dish (diameter 9 cm). Afterwards, holes (diameter 0.6 cm) were punched out and filled with 40 µL of antibiotic solution at the mM concentrations indicated. Pictures were taken after 24 h of incubation in the presence of the antibiotics. (A) kanamycin; (B) allicin; and (C) ampicillin.
Figure 2
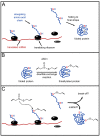
Possible influence of allicin on proteins and protein synthesis. (A) protein synthesis in unstressed conditions. After translation the protein is folded into its final structure. (B) The cysteine residue that is accessible for attack (indicated in red) reacts with allicin via a disulfide exchange-reaction. The cysteine residue that is sterically blocked (indicated in blue) does not react with allicin. (C) According to Cavallito’s hypothesis, allicin may attack cysteine residues on elongating amino acid chains while the protein is still being synthesized and is not fully developed [48]. In this early stage, cysteine residues that are normally blocked for reactions with allicin (compare B) are now potential targets. Possible results may be an abortion of translation or misfolded proteins with reduced or no function.
Scheme 4
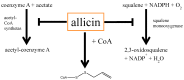
Impact of allicin on cholesterol-metabolism: Allicin was shown to inhibit two important enzymes of the cholesterol-biosynthesis pathway. On the one hand, Gupta and Porter [85] showed that allicin inhibits the squalene-monooxygenase while Focke and coworkers [50] demonstrated an inhibition of acetyl-CoA synthetase, a very early (and thus unspecific) step in cholesterol-biosynthesis. According to its chemistry, one can assume a direct reaction of allicin with the thiol-group of coenzyme A.
Scheme 5
Allicin stimulates lymphocytes by affecting p21ras: Thioallylation that means the binding of an allyl sulfenic acid to the thiol of cysteine118 leads to an activation of p21ras and subsequently to stimulation of ERK1/2 phosphorylation. These processes are crucial for the activation of lymphocytes.
Figure 3
Effect of allicin on seedling development: A. thaliana (Col-0) wild type 48 h after allicin treatment. In this experiment, seeds were set on sterile filter papers which were placed on Murashige & Skoog (MS) medium. After stratification at 4 °C for 48 h, germination and growth took place with the Petri plates tilted to an angle of approx. 20° from vertical to ensure unidirectional root growth according to root gravitropism. After three days of cultivation, filter papers were transferred to MS media that contained different amounts of allicin. Pictures were taken 48 h after treatment. For the sake of clarity only a small representative fraction of seedlings is shown for relevant concentrations.
Figure 4
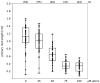
Effect of allicin on elongation of primary root growth: To investigate the effects of allicin on A. thaliana seedlings (accession Col-0), seeds were sown onto sterile filter paper which had been placed on Murashige & Skoog (MS) medium beforehand. After stratification (4 °C, 48 h), the Petri plates were cultivated at an angle approx. 20° from vertical to ensure unidirectional gravitropic root growth. For allicin exposure, the filter papers were transferred onto allicin-containing medium and 48 h after further cultivation, pictures were taken and the root lengths were measured using the software ImageJ [114]. The “boxplot” was prepared with the program PAST [115]; every dot represents the measurement of one root. The two halves of one box represent the 25 and 75 percent quartile, while the horizontal line in the middle shows the median. n = total number of measurements for the given treatment.
Figure 5
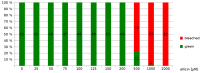
Allicin causes bleaching of seedlings: Sterile filter papers were placed on Murashige & Skoog (MS) medium and were sown with Col-0 A. thaliana wild type seeds. Following stratification (4 °C, 48 h), seeds were allowed to germinate and grow for three days. For allicin treatment, the filter papers were transferred onto MS medium containing different amounts of allicin. Effects on the seedlings were recorded 48 h after allicin treatment. Bleached seedlings showed either partial or complete bleaching, whereas green seedlings showed no signs of bleaching.
Figure 6
Glutathione biosynthesis and function: (A) Cysteine and glutamate are enzymatically linked to γ-glutamylcysteine via the action of glutamate cysteine ligase (GCL). The next step in synthesis is the formation of glutathione itself from cysteine and γ-glutamylcysteine via glutathione synthetase (GS). (B) Glutathione can be oxidized to glutathione dimers (GSSG) and thereby serves to buffer oxidants within the cell. A shift in the equilibrium from GSH towards a higher proportion of GSSG may promote the glutathiolation of free cysteines on protein surfaces, thus shielding these cysteine residues during oxidative stress conditions from over-oxidation. Illustration was redrawn from [116].
Similar articles
- A Comparison of the Antibacterial and Antifungal Activities of Thiosulfinate Analogues of Allicin.
Leontiev R, Hohaus N, Jacob C, Gruhlke MCH, Slusarenko AJ. Leontiev R, et al. Sci Rep. 2018 Apr 30;8(1):6763. doi: 10.1038/s41598-018-25154-9. Sci Rep. 2018. PMID: 29712980 Free PMC article. - Allicin, the Odor of Freshly Crushed Garlic: A Review of Recent Progress in Understanding Allicin's Effects on Cells.
Borlinghaus J, Foerster Née Reiter J, Kappler U, Antelmann H, Noll U, Gruhlke MCH, Slusarenko AJ. Borlinghaus J, et al. Molecules. 2021 Mar 10;26(6):1505. doi: 10.3390/molecules26061505. Molecules. 2021. PMID: 33801955 Free PMC article. Review. - Diallylthiosulfinate (Allicin), a Volatile Antimicrobial from Garlic (Allium sativum), Kills Human Lung Pathogenic Bacteria, Including MDR Strains, as a Vapor.
Reiter J, Levina N, van der Linden M, Gruhlke M, Martin C, Slusarenko AJ. Reiter J, et al. Molecules. 2017 Oct 12;22(10):1711. doi: 10.3390/molecules22101711. Molecules. 2017. PMID: 29023413 Free PMC article. - The human allicin-proteome: S-thioallylation of proteins by the garlic defence substance allicin and its biological effects.
Gruhlke MCH, Antelmann H, Bernhardt J, Kloubert V, Rink L, Slusarenko AJ. Gruhlke MCH, et al. Free Radic Biol Med. 2019 Feb 1;131:144-153. doi: 10.1016/j.freeradbiomed.2018.11.022. Epub 2018 Nov 27. Free Radic Biol Med. 2019. PMID: 30500420 Free PMC article. - Antimicrobial properties of allicin from garlic.
Ankri S, Mirelman D. Ankri S, et al. Microbes Infect. 1999 Feb;1(2):125-9. doi: 10.1016/s1286-4579(99)80003-3. Microbes Infect. 1999. PMID: 10594976 Review.
Cited by
- Ajoene: a natural compound with enhanced antimycobacterial and antibiofilm properties mediated by efflux pump modulation and ROS generation against M. Smegmatis.
Sarangi A, Das BS, Pahuja I, Ojha S, Singh V, Giri S, Bhaskar A, Bhattacharya D. Sarangi A, et al. Arch Microbiol. 2024 Nov 2;206(12):453. doi: 10.1007/s00203-024-04189-9. Arch Microbiol. 2024. PMID: 39487375 - Selenomethionine and Allicin Synergistically Mitigate Intestinal Oxidative Injury by Activating the Nrf2 Pathway.
Liu Y, Lv X, Yuan H, Wang X, Huang J, Wang L. Liu Y, et al. Toxics. 2024 Sep 30;12(10):719. doi: 10.3390/toxics12100719. Toxics. 2024. PMID: 39453138 Free PMC article. - Preparation and Characterization Study of Zein-Sodium Caseinate Nanoparticle Delivery Systems Loaded with Allicin.
Hu L, Zhao P, Wei Y, Lei Y, Guo X, Deng X, Zhang J. Hu L, et al. Foods. 2024 Sep 28;13(19):3111. doi: 10.3390/foods13193111. Foods. 2024. PMID: 39410146 Free PMC article. - Targeting Cancer Hallmarks Using Selected Food Bioactive Compounds: Potentials for Preventive and Therapeutic Strategies.
Talib WH, Abed I, Raad D, Alomari RK, Jamal A, Jabbar R, Alhasan EOA, Alshaeri HK, Alasmari MM, Law D. Talib WH, et al. Foods. 2024 Aug 26;13(17):2687. doi: 10.3390/foods13172687. Foods. 2024. PMID: 39272454 Free PMC article. Review. - Aged garlic therapeutic intervention targeting inflammatory pathways in pathogenesis of bowel disorders.
Liu J. Liu J. Heliyon. 2024 Jul 2;10(14):e33986. doi: 10.1016/j.heliyon.2024.e33986. eCollection 2024 Jul 30. Heliyon. 2024. PMID: 39130474 Free PMC article.
References
- Slusarenko A.J., Patel A., Portz D. Control of plant diseases by natural products: Allicin from garlic as a case study. Eur. J. Plant Pathol. 2008;121:313–322.
- Block E. Garlic and Other Alliums—The Lore and The Science. RSC publishing; Cambridge, UK: 2010.
- Koch H.P., Lawson L.D. Garlic: The Science and Therapeutic Application of Allium sativum L. and Related Species. Williams & Wilkins; Baltimore, MD, USA: 1996.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources