Targets for future clinical trials in Huntington's disease: what's in the pipeline? - PubMed (original) (raw)
Review
. 2014 Sep 15;29(11):1434-45.
doi: 10.1002/mds.26007. Epub 2014 Aug 25.
Affiliations
- PMID: 25155142
- PMCID: PMC4265300
- DOI: 10.1002/mds.26007
Free PMC article
Review
Targets for future clinical trials in Huntington's disease: what's in the pipeline?
Edward J Wild et al. Mov Disord. 2014.
Free PMC article
Abstract
The known genetic cause of Huntington's disease (HD) has fueled considerable progress in understanding its pathobiology and the development of therapeutic approaches aimed at correcting specific changes linked to the causative mutation. Among the most promising is reducing expression of mutant huntingtin protein (mHTT) with RNA interference or antisense oligonucleotides; human trials are now being planned. Zinc-finger transcriptional repression is another innovative method to reduce mHTT expression. Modulation of mHTT phosphorylation, chaperone upregulation, and autophagy enhancement represent attempts to alter cellular homeostasis to favor removal of mHTT. Inhibition of histone deacetylases (HDACs) remains of interest; recent work affirms HDAC4 as a target but questions the assumed centrality of its catalytic activity in HD. Phosphodiesterase inhibition, aimed at restoring synaptic function, has progressed rapidly to human trials. Deranged cellular signaling provides several tractable targets, but specificity and complexity are challenges. Restoring neurotrophic support in HD remains a key potential therapeutic approach. with several approaches being pursued, including brain-derived neurotrophic factor (BDNF) mimesis through tyrosine receptor kinase B (TrkB) agonism and monoclonal antibodies. An increasing understanding of the role of glial cells in HD has led to several new therapeutic avenues, including kynurenine monooxygenase inhibition, immunomodulation by laquinimod, CB2 agonism, and others. The complex metabolic derangements in HD remain under study, but no clear therapeutic strategy has yet emerged. We conclude that many exciting therapeutics are progressing through the development pipeline, and combining a better understanding of HD biology in human patients, with concerted medicinal chemistry efforts, will be crucial for bringing about an era of effective therapies.
Keywords: HDAC inhibition; Huntington's disease; MAPK; gene silencing; glial cells; kynurenine monooxygenase; phosphodiesterase inhibition; therapeutics.
© 2014 The Authors. Movement Disorders published by Wiley Periodicals, Inc. on behalf of International Parkinson and Movement Disorder Society.
Figures
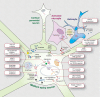
FIG 1
Schematic depicting current priority preclinical therapeutic targets under investigation for Huntington's disease. HTT, huntingtin; KMO, kynurenine monooxygenase; NMDA, N-methyl-D-aspartate; PDE, phosphodiesterase; BDNF, brain-derived neurotrophic factor; HDAC, histone deacetylase; Trk, tropomyosin-related kinase. Adapted from Ross et al.
FIG 2
Schematic illustration of the three main approaches to lowering huntingtin expression. Zinc finger protein (ZFP) therapeutics aim to reduce transcription of the huntingtin gene. Translational repression can be attempted at the pre-mRNA level using DNA-based antisense oligonucleotides (ASOs) or on mature mRNA using short interfering RNA (siRNA) compounds. Different cellular mechanisms degrade the bound mRNA.
Similar articles
- Downregulation of glial genes involved in synaptic function mitigates Huntington's disease pathogenesis.
Onur TS, Laitman A, Zhao H, Keyho R, Kim H, Wang J, Mair M, Wang H, Li L, Perez A, de Haro M, Wan YW, Allen G, Lu B, Al-Ramahi I, Liu Z, Botas J. Onur TS, et al. Elife. 2021 Apr 19;10:e64564. doi: 10.7554/eLife.64564. Elife. 2021. PMID: 33871358 Free PMC article. - Rescue of gene expression by modified REST decoy oligonucleotides in a cellular model of Huntington's disease.
Soldati C, Bithell A, Conforti P, Cattaneo E, Buckley NJ. Soldati C, et al. J Neurochem. 2011 Feb;116(3):415-25. doi: 10.1111/j.1471-4159.2010.07122.x. Epub 2010 Dec 13. J Neurochem. 2011. PMID: 21105876 - Drugging unconventional targets: insights from Huntington's disease.
Yu S, Liang Y, Palacino J, Difiglia M, Lu B. Yu S, et al. Trends Pharmacol Sci. 2014 Feb;35(2):53-62. doi: 10.1016/j.tips.2013.12.001. Epub 2014 Jan 2. Trends Pharmacol Sci. 2014. PMID: 24388390 Review. - Huntingtin Lowering Strategies for Disease Modification in Huntington's Disease.
Tabrizi SJ, Ghosh R, Leavitt BR. Tabrizi SJ, et al. Neuron. 2019 Mar 6;101(5):801-819. doi: 10.1016/j.neuron.2019.01.039. Neuron. 2019. PMID: 30844400 Review. - Advances in Clinical Therapies for Huntington's Disease and the Promise of Multi-Targeted/Functional Drugs Based on Clinicaltrials.gov.
Huang C, Zheng X, Yan S, Zhang Z. Huang C, et al. Clin Pharmacol Ther. 2024 Dec;116(6):1452-1471. doi: 10.1002/cpt.3341. Epub 2024 Jun 11. Clin Pharmacol Ther. 2024. PMID: 38863261 Review.
Cited by
- [Huntington's disease].
Rollnik JD. Rollnik JD. Nervenarzt. 2015 Jun;86(6):725-35. doi: 10.1007/s00115-015-4306-9. Nervenarzt. 2015. PMID: 25940443 Review. German. - Increased irritability, anxiety, and immune reactivity in transgenic Huntington's disease monkeys.
Raper J, Bosinger S, Johnson Z, Tharp G, Moran SP, Chan AWS. Raper J, et al. Brain Behav Immun. 2016 Nov;58:181-190. doi: 10.1016/j.bbi.2016.07.004. Epub 2016 Jul 7. Brain Behav Immun. 2016. PMID: 27395434 Free PMC article. - Huntington's Disease: The Most Curable Incurable Brain Disorder?
Wild EJ. Wild EJ. EBioMedicine. 2016 Jun;8:3-4. doi: 10.1016/j.ebiom.2016.05.023. Epub 2016 May 19. EBioMedicine. 2016. PMID: 27428401 Free PMC article. No abstract available. - Sample enrichment for clinical trials to show delay of onset in huntington disease.
Paulsen JS, Lourens S, Kieburtz K, Zhang Y. Paulsen JS, et al. Mov Disord. 2019 Feb;34(2):274-280. doi: 10.1002/mds.27595. Epub 2019 Jan 14. Mov Disord. 2019. PMID: 30644132 Free PMC article. Clinical Trial. - Pin1-Catalyzed Conformation Changes Regulate Protein Ubiquitination and Degradation.
Jeong J, Usman M, Li Y, Zhou XZ, Lu KP. Jeong J, et al. Cells. 2024 Apr 23;13(9):731. doi: 10.3390/cells13090731. Cells. 2024. PMID: 38727267 Free PMC article. Review.
References
- Ross CA, Tabrizi SJ. Huntington's disease: from molecular pathogenesis to clinical treatment. The Lancet Neurology. 2011;10:83–98. - PubMed
- Bennett CF, Swayze EE. RNA targeting therapeutics: molecular mechanisms of antisense oligonucleotides as a therapeutic platform. Annu Rev Pharmacol Toxicol. 2010;50:259–293. - PubMed
- Martínez T, Wright N, López-Fraga M, Jiménez A, Pañeda C. Silencing human genetic diseases with oligonucleotide-based therapies. Hum Genet. 2013;132:481–493. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical