Evolutionary consequences of intra-patient phage predation on microbial populations - PubMed (original) (raw)
Evolutionary consequences of intra-patient phage predation on microbial populations
Kimberley D Seed et al. Elife. 2014.
Abstract
The impact of phage predation on bacterial pathogens in the context of human disease is not currently appreciated. Here, we show that predatory interactions of a phage with an important environmentally transmitted pathogen, Vibrio cholerae, can modulate the evolutionary trajectory of this pathogen during the natural course of infection within individual patients. We analyzed geographically and temporally disparate cholera patient stool samples from Haiti and Bangladesh and found that phage predation can drive the genomic diversity of intra-patient V. cholerae populations. Intra-patient phage-sensitive and phage-resistant isolates were isogenic except for mutations conferring phage resistance, and moreover, phage-resistant V. cholerae populations were composed of a heterogeneous mix of many unique mutants. We also observed that phage predation can significantly alter the virulence potential of V. cholerae shed from cholera patients. We provide the first molecular evidence for predatory phage shaping microbial community structure during the natural course of infection in humans.
Keywords: OmpU; ToxR; Vibrio cholerae; bacteriophage; cholera; phage.
Copyright © 2014, Seed et al.
Conflict of interest statement
The authors declare that no competing interests exist.
Figures
Figure 1.. The presence of V. cholerae OmpU and ToxR mutants present within and between cholera patients.
(A) Graphical depiction and frequency of OmpU mutants found within a stool sample containing ICP2_2013_A_Haiti phage (108 PFU/ml) from a single Haitian patient (top) and from different patients in Bangladesh (n = 54) (bottom). (B) Predicted membrane topology of mature OmpU generated using Pred-TMBB (Bagos et al., 2004). Locations of amino acid substitutions or insertions carried by V. cholerae clinical isolates are indicated. (C) Graphical depiction and frequency of ToxR mutants found within a stool sample containing ICP2_2011_A (109 PFU/ml) from a single Bangladeshi patient. Amino acid substitutions or nonsense mutations (asterisks) are in orange and duplications are in blue. DOI:
http://dx.doi.org/10.7554/eLife.03497.003
Figure 1—figure supplement 1.. ICP2_2013_A_Haiti is closely related to ICP2 bacteriophages from Bangladesh.
Comparison of ICP2 genomes collected from cholera patients in Dhaka, Bangladesh in 2004 (ICP2), 2006 and 2011, and in Haiti in 2013 using progressiveMauve software. The degree of nucleotide similarity between aligned regions is indicated by the height of the similarity profile (colored blocks), where mauve represents the highly conserved backbone genome and other colors represent segments whose presence varies between isolates. Annotated genes in the ICP2 genome are shown as white boxes, with genes transcribed from the negative strand displaced downward. The numbers above the ICP2 genome show distance in kilobases. DOI:
http://dx.doi.org/10.7554/eLife.03497.004
Figure 1—figure supplement 2.. Identification of OmpU mutants in samples collected at the International Centre for Diarrheal Disease Research, Bangladesh between 2001–2011.
(a) Single V. cholerae O1 El Tor isolates from different stool samples collected within a given year are indicated as closed circles. (b) The number of isolates with the noted mutation is indicated in a given year. If left blank, OmpU was wild-type. DOI:
http://dx.doi.org/10.7554/eLife.03497.005
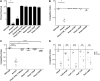
Figure 2.. OmpU expression of OmpU and ToxR mutants and their sensitivity to ICP2.
Outer membrane fractions were prepared from samples matched by equivalent OD600 units. Samples were separated by SDS-PAGE and subjected to Western blot analysis using rabbit polyclonal antisera against OmpU. The sensitivity of each strain to ICP2_2013_A_Haiti is represented as a histogram of the efficiency of plaquing, which is the plaque count ratio of a mutant V. cholerae strain to that of the wild-type strain. The limit of detection for plaque assays was 10−7. DOI:
http://dx.doi.org/10.7554/eLife.03497.006
Figure 3.. The fitness cost of clinically relevant OmpU and ToxR mutations.
(A) Clinically relevant OmpU mutants retain fitness in the presence of bile. *p < 0.05 significantly different means for the compared data sets (Mann–Whitney U Test). (B) OmpU mutants retain competitive fitness in pond water. *p < 0.05 significantly different from wild-type control (Kruskal–Wallis and post hoc Dunn's multiple comparison tests). (C) OmpU mutants have slight competitive fitness defects when serially passaged in Luria–Bertani broth (for ca. 58 generations). *p < 0.05 or ****p < 0.0001 significantly different from wild-type control (Kruskal–Wallis and post hoc Dunn's multiple comparison tests). (D) ToxR mutants are attenuated in vivo using the infant mouse colonization model. **p < 0.01 or ***p < 0.001 significantly different from the in vitro median (Mann–Whitney U Tests). The horizontal bars indicate the median of each data set. Open symbols represent data below the limit of detection. DOI:
http://dx.doi.org/10.7554/eLife.03497.007
Figure 3—figure supplement 1.. Clinical isolates harboring ToxR mutations are severely attenuated for infection.
Competitions indices (CI) in infant mice after 24 hr of infection were determined between each clinical ToxR mutant strain and its isogenic ToxR wild-type revertant strain carrying a lacZ deletion. The lacZ deletion allowed for differentiation of the competing strains upon plating on LB agar plates supplemented with X-gal. Each symbol represents the CI for an individual animal. The horizontal bars indicate the median of each data set. The open symbols represent data below the limit of detection for the ToxR mutant strain. DOI:
http://dx.doi.org/10.7554/eLife.03497.008
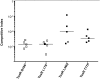
Figure 4.. Phage predation leads to enrichment of OmpU mutant over wild-type in vivo.
Competitive indices (CI) were determined between wild-type Δ_lacZ_ and OmpU G325D in the absence or presence of ICP2_2013_A_Haiti at the multiplicity of infection (MOI) indicated in infant rabbits 12 hr post-infection. Each symbol represents the CI for an individual rabbit and the horizontal lines indicate the median for each condition. The open symbols represent data below the limit of detection for the wild-type strain. **p < 0.01, significantly different from no phage control (Kruskal–Wallis and post hoc Dunn's multiple comparison tests). DOI:
http://dx.doi.org/10.7554/eLife.03497.009
Author response image 1.
Comment in
- Killing the killers.
De Paepe M, Petit MA. De Paepe M, et al. Elife. 2014 Sep 2;3:e04168. doi: 10.7554/eLife.04168. Elife. 2014. PMID: 25182849 Free PMC article. - Bacterial pathogenesis: In-house dining for phage.
Molloy S. Molloy S. Nat Rev Microbiol. 2014 Oct;12(10):658. doi: 10.1038/nrmicro3359. Nat Rev Microbiol. 2014. PMID: 25226053 No abstract available.
Similar articles
- A Tail Fiber Protein and a Receptor-Binding Protein Mediate ICP2 Bacteriophage Interactions with Vibrio cholerae OmpU.
Lim ANW, Yen M, Seed KD, Lazinski DW, Camilli A. Lim ANW, et al. J Bacteriol. 2021 Jun 8;203(13):e0014121. doi: 10.1128/JB.00141-21. Epub 2021 Jun 8. J Bacteriol. 2021. PMID: 33875544 Free PMC article. - Effect of phage on the infectivity of Vibrio cholerae and emergence of genetic variants.
Zahid MS, Udden SM, Faruque AS, Calderwood SB, Mekalanos JJ, Faruque SM. Zahid MS, et al. Infect Immun. 2008 Nov;76(11):5266-73. doi: 10.1128/IAI.00578-08. Epub 2008 Sep 15. Infect Immun. 2008. PMID: 18794293 Free PMC article. - Killing the killers.
De Paepe M, Petit MA. De Paepe M, et al. Elife. 2014 Sep 2;3:e04168. doi: 10.7554/eLife.04168. Elife. 2014. PMID: 25182849 Free PMC article. - Mechanisms of the evolutionary arms race between Vibrio cholerae and Vibriophage clinical isolates.
Yen M, Camilli A. Yen M, et al. Int Microbiol. 2017 Sep;20(3):116-120. doi: 10.2436/20.1501.01.292. Int Microbiol. 2017. PMID: 29446802 Free PMC article. Review. - Survival and proliferation of the lysogenic bacteriophage CTXΦ in Vibrio cholerae.
Fan F, Kan B. Fan F, et al. Virol Sin. 2015 Feb;30(1):19-25. doi: 10.1007/s12250-014-3550-7. Epub 2015 Jan 20. Virol Sin. 2015. PMID: 25613689 Free PMC article. Review.
Cited by
- Modulation of Bacterial Fitness and Virulence Through Antisense RNAs.
Millar JA, Raghavan R. Millar JA, et al. Front Cell Infect Microbiol. 2021 Feb 11;10:596277. doi: 10.3389/fcimb.2020.596277. eCollection 2020. Front Cell Infect Microbiol. 2021. PMID: 33747974 Free PMC article. Review. - Long-term effects of single and combined introductions of antibiotics and bacteriophages on populations of Pseudomonas aeruginosa.
Torres-Barceló C, Franzon B, Vasse M, Hochberg ME. Torres-Barceló C, et al. Evol Appl. 2016 Feb 18;9(4):583-95. doi: 10.1111/eva.12364. eCollection 2016 Apr. Evol Appl. 2016. PMID: 27099623 Free PMC article. - Niche adaptation limits bacteriophage predation of Vibrio cholerae in a nutrient-poor aquatic environment.
Silva-Valenzuela CA, Camilli A. Silva-Valenzuela CA, et al. Proc Natl Acad Sci U S A. 2019 Jan 29;116(5):1627-1632. doi: 10.1073/pnas.1810138116. Epub 2019 Jan 11. Proc Natl Acad Sci U S A. 2019. PMID: 30635420 Free PMC article. - Sequential Combined Effect of Phages and Antibiotics on the Inactivation of Escherichia coli.
Lopes A, Pereira C, Almeida A. Lopes A, et al. Microorganisms. 2018 Dec 5;6(4):125. doi: 10.3390/microorganisms6040125. Microorganisms. 2018. PMID: 30563133 Free PMC article. - The disparate effects of bacteriophages on antibiotic-resistant bacteria.
Torres-Barceló C. Torres-Barceló C. Emerg Microbes Infect. 2018 Oct 10;7(1):168. doi: 10.1038/s41426-018-0169-z. Emerg Microbes Infect. 2018. PMID: 30302018 Free PMC article. Review.
References
- Chin CS, Sorenson J, Harris JB, Robins WP, Charles RC, Jean-Charles RR, Bullard J, Webster DR, Kasarskis A, Peluso P, Paxinos EE, Yamaichi Y, Calderwood SB, Mekalanos JJ, Schadt EE, Waldor MK. 2011. The origin of the Haitian cholera outbreak strain. The New England Journal of Medicine 364:33–42. doi: 10.1056/NEJMoa1012928 - DOI - PMC - PubMed
- Cravioto A, Lanata CF, Lantagne DS, Nair GB. 2011. Final report of the independent panel of experts on the cholera outbreak in Haiti. Available at: http://www.un.org/News/dh/infocus/haiti/UN-cholera-report-final.pdf
Publication types
MeSH terms
Substances
Grants and funding
- AI099243/AI/NIAID NIH HHS/United States
- K08 AI089721/AI/NIAID NIH HHS/United States
- R37 AI055058/AI/NIAID NIH HHS/United States
- AI055058/AI/NIAID NIH HHS/United States
- R01 AI099243/AI/NIAID NIH HHS/United States
- T32 AI007422/AI/NIAID NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- R01 AI055058/AI/NIAID NIH HHS/United States
- R01 AI103055/AI/NIAID NIH HHS/United States
- AI089721/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical