Structural characterization by cross-linking reveals the detailed architecture of a coatomer-related heptameric module from the nuclear pore complex - PubMed (original) (raw)
Structural characterization by cross-linking reveals the detailed architecture of a coatomer-related heptameric module from the nuclear pore complex
Yi Shi et al. Mol Cell Proteomics. 2014 Nov.
Abstract
Most cellular processes are orchestrated by macromolecular complexes. However, structural elucidation of these endogenous complexes can be challenging because they frequently contain large numbers of proteins, are compositionally and morphologically heterogeneous, can be dynamic, and are often of low abundance in the cell. Here, we present a strategy for the structural characterization of such complexes that has at its center chemical cross-linking with mass spectrometric readout. In this strategy, we isolate the endogenous complexes using a highly optimized sample preparation protocol and generate a comprehensive, high-quality cross-linking dataset using two complementary cross-linking reagents. We then determine the structure of the complex using a refined integrative method that combines the cross-linking data with information generated from other sources, including electron microscopy, X-ray crystallography, and comparative protein structure modeling. We applied this integrative strategy to determine the structure of the native Nup84 complex, a stable hetero-heptameric assembly (∼ 600 kDa), 16 copies of which form the outer rings of the 50-MDa nuclear pore complex (NPC) in budding yeast. The unprecedented detail of the Nup84 complex structure reveals previously unseen features in its pentameric structural hub and provides information on the conformational flexibility of the assembly. These additional details further support and augment the protocoatomer hypothesis, which proposes an evolutionary relationship between vesicle coating complexes and the NPC, and indicates a conserved mechanism by which the NPC is anchored in the nuclear envelope.
© 2014 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures
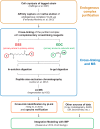
Fig. 1.
CX-MS integrative pipeline for the structural characterization of endogenous complexes. The workflow of the cross-linking and mass spectrometric analysis of endogenous Nup84 complex is summarized. The endogenously tagged protein complex is affinity purified from cell cryolysis, natively eluted, and cross-linked by two complementary cross-linkers, DSS and EDC (14, 70). The cross-linked complex is then subjected to in-solution and in-gel digestion, and the resulting peptide mixtures are fractionated using peptide size exclusion chromatography (71). The cross-linked peptides were analyzed by a Velos Orbitrap mass spectrometer using high-resolution HCD and searched by pLink (40). All software-produced identifications were manually inspected (see “Experimental Procedures”) (44).
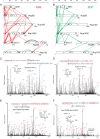
Fig. 2.
CX-MS analysis of the Nup84 complex. Cross-linking maps of the Nup84 complex with either DSS cross-linker (A) or EDC cross-linker (B). Straight lines in red connecting residues from different subunits represent DSS intersubunit cross-links, and straight lines in green represent EDC intersubunit cross-links. Curved, dotted lines represent intrasubunit cross-links. Only intrasubunit cross-links between residues more than 40 positions apart are shown. C, a high-resolution HCD MS/MS spectrum (m/z = 1096.169, z = 6) of the intersubunit DSS cross-link connecting Nup85 residue 30 (lysine) and Seh1 residue 1 (N-terminal methionine). The methionines are oxidized. F* indicates an immonium ion of phenylalanine that is frequently observed in HCD spectra. b and y ion series including their charge states are labeled. The fragment of y20 is zoomed, and the mass accuracy is labeled in parts per million. D, a high-resolution HCD MS/MS spectrum (m/z 1044.139, z = 6) of the intersubunit EDC cross-link connecting Nup85 residue 17 and Seh1 residue 1. The spectrum is labeled as in C. The fragment of y20 is zoomed, and the mass accuracy is labeled in parts per million. E, a high-resolution HCD MS/MS spectrum (m/z = 903.317, z = 7) of the intrasubunit DSS cross-link connecting Nup133 residue 506 and Nup133 residue 59. F* and Y* indicate immonium ions of phenylalanine and tyrosine that are common for HCD fragmentations. The fragment of b14 is zoomed, and the mass accuracy is labeled in parts per million. F, a high-resolution HCD MS/MS spectrum (m/z = 917.327, z = 6) of the intrasubunit EDC cross-link connecting Nup133 residue 506 and residue 562. F* and Y* indicate immonium ions of phenylalanine and tyrosine that are common to HCD fragmentations. The fragment of y16 is zoomed, and the mass accuracy is labeled in parts per million. Charge states of the fragments in the high-mass region of this spectrum (i.e. 1100–1300 m/z) are not resolved.
Fig. 3.
Distance distribution of the cross-links and their mapping on the available crystal structures. A, we mapped the Euclidean Cα–Cα distances between the cross-linked residues onto several domains of the Nup84 complex proteins with available crystal structures (49, 50). The Cα–Cα distance distributions of the cross-linked residues are shown for both DSS and EDC cross-links. All the measured DSS cross-links fall within the expected maximum threshold of ∼30 Å (72), and the great majority of EDC cross-links fall within the expected threshold of 17 Å. B, both DSS (in red) and EDC (in green) cross-links are mapped on the crystallographic structure of the Sec13–Nup145c–Nup84 (PDB code 3IKO (50)). The EDC and DSS cross-links provide complementary spatial information.
Fig. 4.
The four-stage scheme for integrative structure determination of the Nup84 complex. Our integrative approach to determining the Nup84 complex structure proceeds through four stages (7, 13, 14, 43): (1) gathering of data, (2) representation of subunits and translation of the data into spatial restraints, (3) configurational sampling to produce an ensemble of models that satisfies the restraints, and (4) analysis of the ensemble. The modeling protocol (i.e. stages 2, 3, and 4) was scripted using the Python Modeling Interface, version be72c15, a library for modeling macromolecular complexes based on our open-source Integrative Modeling Platform (IMP) package, version 829c3f0 (44).
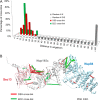
Fig. 5.
Correlation between the number of cross-links and the accuracy of dimer models. A, localization density maps of the Nup84 subunits (solid contour surfaces; Fig. 6_A_) and position of the Nup145c–Sec13 crystallographic dimer (PDB code 3IKO (50)). B, total scores (i.e. the sum of excluded volume and cross-link restraint scores) are plotted as a function of the Cα dRMSD of the Nup145c–Sec13 dimer models with respect to the crystallographic dimer; as the number of cross-links increases, the ensemble of models is enriched in accurate structures (i.e. low dRMSD models, left of dotted line). C, accuracy of dimer models as a function of the number and type of cross-links. Each symbol displays the first and third quartile (lower and upper side of the boxes), median (red line), and minimum and maximum (lower and upper limit of the dashed whiskers, respectively) of the Cα dRMSD with respect to the crystallographic structure for the 100 best-scoring models. The median and the spread (i.e. difference between the first and third quartile) of the distribution are measures of the accuracy and precision of the ensemble of models, respectively.
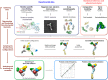
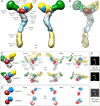
Fig. 6.
The Nup84 complex molecular architecture revealed by the CX-MS integrative pipeline. The localization density maps of the Nup84 subunits (solid contour surfaces) and the entire complex (transparent surfaces) were computed and contoured at the threshold of 2.5 times their volumes estimated from sequence (
supplemental Table S6
) (A through C). A, front and back views of the localization density maps of the Nup84 subunits and the entire complex. B, a representative single Nup84 complex structure (colored ribbon) is shown along with the localization density maps of the individual subunits. C, the localization density maps of the two dominant clusters computed on the hub region (Nup120-CTD, Nup85, Nup145c, Sec13, and Seh1) are shown, along with the representative single structures of the hub region for each of the two clusters, from multiple viewing points. D, the positions of Sec13 and Seh1 are presented for each of the two clusters. E, the representative model projections in each of the two clusters are shown, along with the EM class average (14).
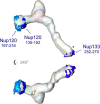
Fig. 7.
The putative membrane-interacting ALPS motifs. One putative ALPS motif in Nup133 (252–270) and two in Nup120 (135–152 and 197–216) are mapped on the Nup84 complex. All three putative ALPS motifs are localized to peripheral positions of the Nup84 complex.
Similar articles
- Integrative structure-function mapping of the nucleoporin Nup133 suggests a conserved mechanism for membrane anchoring of the nuclear pore complex.
Kim SJ, Fernandez-Martinez J, Sampathkumar P, Martel A, Matsui T, Tsuruta H, Weiss TM, Shi Y, Markina-Inarrairaegui A, Bonanno JB, Sauder JM, Burley SK, Chait BT, Almo SC, Rout MP, Sali A. Kim SJ, et al. Mol Cell Proteomics. 2014 Nov;13(11):2911-26. doi: 10.1074/mcp.M114.040915. Epub 2014 Aug 19. Mol Cell Proteomics. 2014. PMID: 25139911 Free PMC article. - Three-dimensional structure and flexibility of a membrane-coating module of the nuclear pore complex.
Kampmann M, Blobel G. Kampmann M, et al. Nat Struct Mol Biol. 2009 Jul;16(7):782-8. doi: 10.1038/nsmb.1618. Epub 2009 Jun 7. Nat Struct Mol Biol. 2009. PMID: 19503077 Free PMC article. - Structure and Function of the Nuclear Pore Complex Cytoplasmic mRNA Export Platform.
Fernandez-Martinez J, Kim SJ, Shi Y, Upla P, Pellarin R, Gagnon M, Chemmama IE, Wang J, Nudelman I, Zhang W, Williams R, Rice WJ, Stokes DL, Zenklusen D, Chait BT, Sali A, Rout MP. Fernandez-Martinez J, et al. Cell. 2016 Nov 17;167(5):1215-1228.e25. doi: 10.1016/j.cell.2016.10.028. Epub 2016 Nov 10. Cell. 2016. PMID: 27839866 Free PMC article. - Characterization of the membrane-coating Nup84 complex: paradigm for the nuclear pore complex structure.
Debler EW, Hsia KC, Nagy V, Seo HS, Hoelz A. Debler EW, et al. Nucleus. 2010 Mar-Apr;1(2):150-7. doi: 10.4161/nucl.1.2.11120. Epub 2010 Jan 3. Nucleus. 2010. PMID: 21326946 Free PMC article. Review. - Membrane-coating lattice scaffolds in the nuclear pore and vesicle coats: commonalities, differences, challenges.
Leksa NC, Schwartz TU. Leksa NC, et al. Nucleus. 2010 Jul-Aug;1(4):314-8. doi: 10.4161/nucl.1.4.11798. Epub 2010 Mar 12. Nucleus. 2010. PMID: 21327078 Free PMC article. Review.
Cited by
- Improving integrative 3D modeling into low- to medium-resolution electron microscopy structures with evolutionary couplings.
McCafferty CL, Taylor DW, Marcotte EM. McCafferty CL, et al. Protein Sci. 2021 May;30(5):1006-1021. doi: 10.1002/pro.4067. Epub 2021 Apr 9. Protein Sci. 2021. PMID: 33759266 Free PMC article. - Implications of a multiscale structure of the yeast nuclear pore complex.
Akey CW, Echeverria I, Ouch C, Nudelman I, Shi Y, Wang J, Chait BT, Sali A, Fernandez-Martinez J, Rout MP. Akey CW, et al. Mol Cell. 2023 Sep 21;83(18):3283-3302.e5. doi: 10.1016/j.molcel.2023.08.025. Mol Cell. 2023. PMID: 37738963 Free PMC article. - Anticipating innovations in structural biology.
Berman HM, Lawson CL, Vallat B, Gabanyi MJ. Berman HM, et al. Q Rev Biophys. 2018 Jan;51:e8. doi: 10.1017/S0033583518000057. Q Rev Biophys. 2018. PMID: 30912485 Free PMC article. Review. - Exploring Spacer Arm Structures for Designs of Asymmetric Sulfoxide-Containing MS-Cleavable Cross-Linkers.
Yu C, Novitsky EJ, Cheng NW, Rychnovsky SD, Huang L. Yu C, et al. Anal Chem. 2020 Apr 21;92(8):6026-6033. doi: 10.1021/acs.analchem.0c00298. Epub 2020 Mar 31. Anal Chem. 2020. PMID: 32202417 Free PMC article. - Structural Biology in the Multi-Omics Era.
McCafferty CL, Verbeke EJ, Marcotte EM, Taylor DW. McCafferty CL, et al. J Chem Inf Model. 2020 May 26;60(5):2424-2429. doi: 10.1021/acs.jcim.9b01164. Epub 2020 Mar 10. J Chem Inf Model. 2020. PMID: 32129623 Free PMC article.
References
- Gavin A. C., Aloy P., Grandi P., Krause R., Boesche M., Marzioch M., Rau C., Jensen L. J., Bastuck S., Dumpelfeld B., Edelmann A., Heurtier M. A., Hoffman V., Hoefert C., Klein K., Hudak M., Michon A. M., Schelder M., Schirle M., Remor M., Rudi T., Hooper S., Bauer A., Bouwmeester T., Casari G., Drewes G., Neubauer G., Rick J. M., Kuster B., Bork P., Russell R. B., Superti-Furga G. (2006) Proteome survey reveals modularity of the yeast cell machinery. Nature 440, 631–636 - PubMed
- Krogan N. J., Cagney G., Yu H., Zhong G., Guo X., Ignatchenko A., Li J., Pu S., Datta N., Tikuisis A. P., Punna T., Peregrin-Alvarez J. M., Shales M., Zhang X., Davey M., Robinson M. D., Paccanaro A., Bray J. E., Sheung A., Beattie B., Richards D. P., Canadien V., Lalev A., Mena F., Wong P., Starostine A., Canete M. M., Vlasblom J., Wu S., Orsi C., Collins S. R., Chandran S., Haw R., Rilstone J. J., Gandi K., Thompson N. J., Musso G., St Onge P., Ghanny S., Lam M. H., Butland G., Altaf-Ul A. M., Kanaya S., Shilatifard A., O'Shea E., Weissman J. S., Ingles C. J., Hughes T. R., Parkinson J., Gerstein M., Wodak S. J., Emili A., Greenblatt J. F. (2006) Global landscape of protein complexes in the yeast Saccharomyces cerevisiae. Nature 440, 637–643 - PubMed
- Robinson C. V., Sali A., Baumeister W. (2007) The molecular sociology of the cell. Nature 450, 973–982 - PubMed
- Alber F., Forster F., Korkin D., Topf M., Sali A. (2008) Integrating diverse data for structure determination of macromolecular assemblies. Annu. Rev. Biochem. 77, 443–477 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 GM083960/GM/NIGMS NIH HHS/United States
- P41 GM103314/GM/NIGMS NIH HHS/United States
- U54 GM103511/GM/NIGMS NIH HHS/United States
- U01 GM098256/GM/NIGMS NIH HHS/United States
- P41 GM109824/GM/NIGMS NIH HHS/United States
- R01 GM062427/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases