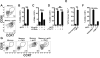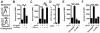T cell memory. Resident memory CD8 T cells trigger protective innate and adaptive immune responses - PubMed (original) (raw)
T cell memory. Resident memory CD8 T cells trigger protective innate and adaptive immune responses
Jason M Schenkel et al. Science. 2014.
Abstract
The pathogen recognition theory dictates that, upon viral infection, the innate immune system first detects microbial products and then responds by providing instructions to adaptive CD8 T cells. Here, we show in mice that tissue resident memory CD8 T cells (T(RM) cells), non-recirculating cells located at common sites of infection, can achieve near-sterilizing immunity against viral infections by reversing this flow of information. Upon antigen resensitization within the mouse female reproductive mucosae, CD8(+) T(RM) cells secrete cytokines that trigger rapid adaptive and innate immune responses, including local humoral responses, maturation of local dendritic cells, and activation of natural killer cells. This provided near-sterilizing immunity against an antigenically unrelated viral infection. Thus, CD8(+) T(RM) cells rapidly trigger an antiviral state by amplifying receptor-derived signals from previously encountered pathogens.
Copyright © 2014, American Association for the Advancement of Science.
Figures
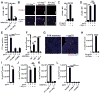
Fig 1. TRM reactivation induces memory CD8 T cell and B cell recruitment through IFNγ-dependent VCAM-1 upregulation
(A) VCAM-1 mean fluorescence intensity (MFI) on CD31+ vascular endothelium in the FRT 12h after OT-I Ifng-/- or OT-I Ifng+/+ TRM were reactivated by SIINFEKL peptide deposited t.c. (B) Representative images of VCAM-1 (red) and CD31 (blue) staining. 12 hours after t.c. challenge, VCAM-1 expression was quantified on FRT vascular endothelium after (C) P14 immune chimeras were t.c. challenged with either VV-gp33, VV-OVA or left untreated or (D) when P14 immune chimeras were injected with anti-Thy1.1 antibody or left untreated 5 days prior to t.c. deposition of gp33 peptide. (E) OT-I CD8 T cells were transferred i.v. into P14 immune chimeras that were treated with anti-VCAM-1, anti-CD49d or isotype control antibody and were then challenged with gp33 t.c. 48h later, OT-I T cells per coronal section were enumerated. (F) B cells in the FRT were enumerated 48h after t.c. gp33 peptide challenge of P14 immune chimeras, and (G) shows a representative image. B cells in the FRT were also enumerated in (H) P14 immune chimeras that were t.c. challenged with either VV-gp33, VV-OVA or left untreated, in (I) LCMV immune mice that never received P14 cells, and (J) in P14 immune chimeras that were injected with anti-Thy1.1 antibody 5d prior to t.c. gp33 peptide challenge. (K) B cells within the FRT of OT-I Ifng-/- or OT-I Ifng+/+ immune chimeras were enumerated 48h after t.c. SIINFEKL challenge. (L) B cells in the FRT were quantified 48h after P14 immune chimeras were challenged t.c. with gp33 peptide in the presence of VCAM-1 or CD49d blocking antibodies. Each experiment shown includes 3-6 mice and data is representative of 2-3 independent experiments. Scale bars=20μm. *=p<0.05, **=p<0.01, ***=p<.001, unpaired two-tailed _t_-test, error bars indicate mean±SEM.
Fig 2. TRM reactivation induces DC maturation
CD86 and CCR7 expression was evaluated on CD11c+/MHC-II+ DC in the FRT 12h after t.c challenge of (A) P14 immune chimeras challenged with gp33 peptide, (B) LCMV immune mice (that never received P14 cells) challenged with gp33 peptide, (C) P14 immune chimeras that were t.c. challenged with either VV-gp33 or VV-OVA, or (D) P14 immune chimeras that were injected i.p. with 1 μg of anti-Thy1.1 antibody five days prior to gp33 challenge. (E) Intracellular TNFα expression was evaluated in P14 CD8 T cells from the FRT by flow cytometry 12h after t.c. gp33 challenge. (F&G) DC phenotype was evaluated as in (A), but the indicated mice were pretreated with TNFα blocking antibody. Representative of 2-3 experiments totaling 6-14 mice/group. *=p<0.05, **=p<0.01, ***=p<.001, unpaired two-tailed _t_-test, error bars indicated mean±SEM.
Fig 3. TRM reactivation induces NK cell activation
(A) P14 immune chimeras were challenged t.c. with gp33 to reactivate TRM. 12h later, intracellular granzyme B expression was evaluated in bystander CD8 T cells (P14 CD8 T cells were excluded from analysis) and NK cells isolated from the FRT (grey line=without gp33 challenge, black=gp33 challenge). Intracellular granzyme B expression within NK cells isolated from the FRT was evaluated 12h after (B) P14 immune chimeras were t.c. challenged with either VV-gp33 or VV-OVA or left untreated or (C) P14 immune chimeras were previously treated with anti-Thy1.1 antibody before t.c. gp33 peptide challenge. (D) 12h after t.c. gp33 peptide challenge, intracellular IL-2 expression by P14 CD8 T cells isolated from the FRT of P14 immune chimeras was evaluated. Intracellular granzyme B expression by (E) NK cells and (F) CD8 T cells isolated from the FRT of P14 chimeras 12h after gp33 peptide challenge when mice were pre-treated with IL-2Rβ blocking antibody. n=3, representative of 3 experiments. *=p<0.05, **=p<0.01, ***=p<.001,unpaired two-tailed _t_-test,mean±SEM.
Fig 4. TRM reactivation induces antiviral state
(A) P14 immune chimeras or control naïve mice were challenged t.c. with 4×106 PFU of antigenically unrelated VV-OVA in the presence or absence of gp33 reactivating peptide and/or IFNγ, TNFα, and IL-2Rβ blocking antibodies. Two days later, viral titers were evaluated by plaque assay from homogenized FRT. Data pooled from two independent experiments totaling 6-11 mice per group. (B) As in A, however gp33 peptide was delivered 12h prior to viral challenge. Data pooled from two independent experiments totaling 8 or 9 mice per group. (C) LCMV immune mice (without P14 transfer) were transcervically challenged with 1×106 PFU of VV-OVA in the presence or absence of gp33 peptide. Two days after infection, viral titers were evaluated. Data pooled from two independent experiments totaling 6 mice per group. *=p<.05, **=p<.01,***=p<.001, unpaired two-tailed _t_-test, mean±SEM.
Comment in
- Immunology. A neighborhood watch upholds local immune protection.
Carbone FR, Gebhardt T. Carbone FR, et al. Science. 2014 Oct 3;346(6205):40-1. doi: 10.1126/science.1259925. Epub 2014 Oct 2. Science. 2014. PMID: 25278601 No abstract available.
Similar articles
- Memory CD8+ T Cells: Orchestrators and Key Players of Innate Immunity?
Lauvau G, Goriely S. Lauvau G, et al. PLoS Pathog. 2016 Sep 1;12(9):e1005722. doi: 10.1371/journal.ppat.1005722. eCollection 2016 Sep. PLoS Pathog. 2016. PMID: 27584152 Free PMC article. - Tissue-resident lymphocytes: from adaptive to innate immunity.
Sun H, Sun C, Xiao W, Sun R. Sun H, et al. Cell Mol Immunol. 2019 Mar;16(3):205-215. doi: 10.1038/s41423-018-0192-y. Epub 2019 Jan 11. Cell Mol Immunol. 2019. PMID: 30635650 Free PMC article. Review. - Tissue-Resident-Memory CD8+ T Cells Bridge Innate Immune Responses in Neighboring Epithelial Cells to Control Human Genital Herpes.
Peng T, Phasouk K, Sodroski CN, Sun S, Hwangbo Y, Layton ED, Jin L, Klock A, Diem K, Magaret AS, Jing L, Laing K, Li A, Huang ML, Mertens M, Johnston C, Jerome KR, Koelle DM, Wald A, Knipe DM, Corey L, Zhu J. Peng T, et al. Front Immunol. 2021 Sep 6;12:735643. doi: 10.3389/fimmu.2021.735643. eCollection 2021. Front Immunol. 2021. PMID: 34552595 Free PMC article. - Rapid innate control of antigen abrogates adaptive immunity.
Pembroke TP, Gallimore AM, Godkin A. Pembroke TP, et al. Immunology. 2013 Apr;138(4):293-7. doi: 10.1111/imm.12048. Immunology. 2013. PMID: 23198899 Free PMC article. Review.
Cited by
- IL-12 drives the expression of the inhibitory receptor NKG2A on human tumor-reactive CD8 T cells.
Fesneau O, Samson KA, Rosales W, Jones B, Moudgil T, Fox BA, Rajamanickam V, Duhen T. Fesneau O, et al. Nat Commun. 2024 Nov 18;15(1):9988. doi: 10.1038/s41467-024-54420-w. Nat Commun. 2024. PMID: 39557863 - Nasal mRNA Nanovaccine with Key Activators of Dendritic and MAIT Cells for Effective Against Lung Tumor Metastasis in Mice Model.
Li A, Cai X, Li D, Yu Y, Liu C, Shen J, You J, Qiao J, Wang F. Li A, et al. Int J Nanomedicine. 2024 Nov 8;19:11479-11497. doi: 10.2147/IJN.S479741. eCollection 2024. Int J Nanomedicine. 2024. PMID: 39534380 Free PMC article. - Rationally designed multimeric nanovaccines using icosahedral DNA origami for display of SARS-CoV-2 receptor binding domain.
Feng Q, Cheng K, Zhang L, Wang D, Gao X, Liang J, Liu G, Ma N, Xu C, Tang M, Chen L, Wang X, Ma X, Zou J, Shi Q, Du P, Wang Q, Wang H, Nie G, Zhao X. Feng Q, et al. Nat Commun. 2024 Nov 6;15(1):9581. doi: 10.1038/s41467-024-53937-4. Nat Commun. 2024. PMID: 39505890 Free PMC article. - Evaluation of adenoviral vector Ad19a encoding RSV-F as novel vaccine against respiratory syncytial virus.
Fuchs J, Hübner J, Schmidt A, Irrgang P, Maier C, Vieira Antão A, Oltmanns F, Thirion C, Lapuente D, Tenbusch M. Fuchs J, et al. NPJ Vaccines. 2024 Oct 29;9(1):205. doi: 10.1038/s41541-024-01001-z. NPJ Vaccines. 2024. PMID: 39472590 Free PMC article. - T-Cell Immune Responses to SARS-CoV-2 Infection and Vaccination.
Notarbartolo S. Notarbartolo S. Vaccines (Basel). 2024 Sep 30;12(10):1126. doi: 10.3390/vaccines12101126. Vaccines (Basel). 2024. PMID: 39460293 Free PMC article. Review.
References
- Medzhitov R, Janeway C. Innate immunity. N Engl J Med. 2000;343:338–344. - PubMed
- Russell JH, Ley TJ. Lymphocyte mediated cytotoxicity. Annu Rev Immunol. 2002;20:323–370. - PubMed
- Mueller SN, Gebhardt T, Carbone FR, Heath WR. Memory T cell subsets, migration patterns, and tissue residence. Annu Rev Immunol. 2013;31:137–161. - PubMed
- Masopust D, Schenkel JM. The integration of T cell migration, differentiation and function. Nat Rev Immunol. 2013;13:309–320. - PubMed
- Gebhardt T, et al. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat Immunol. 2009;10:524–530. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R37 AI084913/AI/NIAID NIH HHS/United States
- T32 AI007313/AI/NIAID NIH HHS/United States
- R01 AI084913/AI/NIAID NIH HHS/United States
- DP2 OD006467/OD/NIH HHS/United States
- DP2-OD-006467/OD/NIH HHS/United States
- F30 DK100159/DK/NIDDK NIH HHS/United States
- F30DK100159/DK/NIDDK NIH HHS/United States
- T32AI007313/AI/NIAID NIH HHS/United States
- R01AI084913/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials