Cross-talk among myeloid-derived suppressor cells, macrophages, and tumor cells impacts the inflammatory milieu of solid tumors - PubMed (original) (raw)
Cross-talk among myeloid-derived suppressor cells, macrophages, and tumor cells impacts the inflammatory milieu of solid tumors
Daniel W Beury et al. J Leukoc Biol. 2014 Dec.
Abstract
MDSC and macrophages are present in most solid tumors and are important drivers of immune suppression and inflammation. It is established that cross-talk between MDSC and macrophages impacts anti-tumor immunity; however, interactions between tumor cells and MDSC or macrophages are less well studied. To examine potential interactions between these cells, we studied the impact of MDSC, macrophages, and four murine tumor cell lines on each other, both in vitro and in vivo. We focused on IL-6, IL-10, IL-12, TNF-α, and NO, as these molecules are produced by macrophages, MDSC, and many tumor cells; are present in most solid tumors; and regulate inflammation. In vitro studies demonstrated that MDSC-produced IL-10 decreased macrophage IL-6 and TNF-α and increased NO. IL-6 indirectly regulated MDSC IL-10. Tumor cells increased MDSC IL-6 and vice versa. Tumor cells also increased macrophage IL-6 and NO and decreased macrophage TNF-α. Tumor cell-driven macrophage IL-6 was reduced by MDSC, and tumor cells and MDSC enhanced macrophage NO. In vivo analysis of solid tumors identified IL-6 and IL-10 as the dominant cytokines and demonstrated that these molecules were produced predominantly by stromal cells. These results suggest that inflammation within solid tumors is regulated by the ratio of tumor cells to MDSC and macrophages and that interactions of these cells have the potential to alter significantly the inflammatory milieu within the tumor microenvironment.
Keywords: IL-10; IL-6; cancer; cytokines; nitric oxide; tumor microenvironment.
© 2014 Society for Leukocyte Biology.
Figures
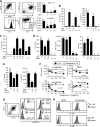
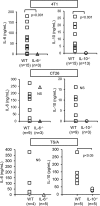
Figure 1.. IL-6 and IL-10 produced by host cells enhance primary tumor growth and decrease survival time.
WT, IL-6−/−, and IL-10−/− BALB/c mice were inoculated with (A) 4T1 or (B) CT26 tumor cells and monitored for tumor diameter, survival, and tumor engraftment. Mice in the WT versus IL-6−/− graphs and WT versus IL-10−/− graphs (tumor diameter and percent survival) were inoculated with 1 × 105 and 7000 4T1 cells, respectively. Mice in the engraftment graph were inoculated with 1 × 105 4T1 cells. All CT26 inoculations were 5 × 105 cells. For tumor engraftment, n = 7 for each 4T1 group; n = 6 for each CT26 group. Statistical significance was tested by Mann-Whitney (tumor growth) or log-rank test (survival). Data are pooled from three independent experiments.
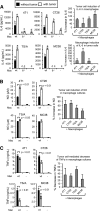
Figure 2.. Cross-talk between MDSC and macrophages regulates production of IL-10, IL-6, and NO.
(A) Peritoneal macrophages from healthy mice and MDSC from tumor-bearing mice were stained with mAb to CD11b, F4/80, Gr1, Ly6C, and/or Ly6G and analyzed by flow cytometry. MDSC from WT, IL-10−/−, and IL-6−/− BALB/c mice with 4T1 tumors were assayed for their ability to suppress the antigen-driven activation of peptide-specific, MHC-restricted, transgenic CD4+ (DO11.10) and CD8+ (Clone 4) T cells. (B–D) 4T1-induced MDSC and peritoneal macrophages (Mac) from WT, IL-10−/− (10−/−), or IL-6−/− (6−/−) BALB/c mice were cocultured, and supernatants were assayed for IL-10, IL-6, and NO. (B) Macrophages enhance MDSC IL-10, and MDSC decrease macrophage IL-6. (C) IL-6 decreases MDSC IL-10. (D) IL-10 production by MDSC decreases macrophage IL-6 and TNF-α and increases macrophage NO. (E) Neutralizing antibodies to IL-10 prevent the down-regulation of macrophage IL-6 and IL-12. (F) Exogenous IL-10 decreases MDSC and macrophage TNF-α, decreases macrophage IL-6, and enhances macrophage NO. Macrophages or MDSC were cultured with IL-10 or denatured IL-10. (G) Macrophages activate STAT3 in response to IL-10. Macrophages (left) were cultured for 5 min in the presence or absence of exogenous IL-10 (middle) or with supernatants (media) from MDSC-macrophage cocultures (right), subsequently fixed and permeabilized, and then stained for F4/80, CD11b, and pSTAT3. (H) Macrophages and MDSC express the receptors for IL-10 and IL-6, respectively. (A–H) Data are from one of two, 30, three, five, four, three, two, and two experiments, respectively. Statistical significance was determined by (A–E) Tukey's HSD test and (F) the Mann-Whitney test. Different lower case letters above each value indicate that those values are statistically, significantly different; values that share the same lowercase letter are not statistically, significantly different.
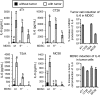
Figure 3.. Tumor cells induce MDSC to produce IL-6 and vice versa.
WT or IL-6−/− 4T1-induced MDSC were cultured with or without 4T1, CT26, TS/A, or MC38 tumor cells, and the supernatants were assayed for IL-6 by ELISA. One of three independent experiments (left four graphs); average percent increase of three independent experiments comparing tumor cells with IL-6−/− and WT MDSC (right two graphs). Statistical significance for the independent experiments was determined by Tukey's HSD test.
Figure 4.. Tumor cells induce macrophages to produce IL-6 and NO but decrease macrophage TNF-α.
WT or IL-6−/− macrophages were cultured with or without 4T1, CT26, TS/A, or MC38 tumor cells, and the supernatants were assayed for (A) IL-6, (B) NO, or (C) TNF-α. Representative data (left graphs) from one of four, three, and four independent experiments, respectively. Average percent change of pooled data from all experiments (right graphs). (A) Comparison of tumor cells with IL-6−/− and WT macrophages. (B and C) Comparison of tumor cells with WT macrophages. (A–C) Statistical significance was determined by _t_-test.
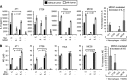
Figure 5.. MDSC decrease tumor cell-mediated enhancement of IL-6 and increase tumor cell-mediated enhancement of macrophage NO.
WT macrophages were cultured with or without 4T1-induced MDSC and/or 4T1, CT26, TS/A, or MC38 tumor cells. Supernatants were assayed for (A) IL-6 and (B) NO. (A and B, left four graphs of each) One of three independent experiments. (Right) Average of the three independent experiments. (A and B) Statistical significance was determined by _t_-test.
Figure 6.. Host cells are the dominant producers of IL-6 and IL-10 in the tumor microenvironment.
Eight- to 10-mm diameter 4T1, CT26, and TS/A tumors were excised from WT, IL-6−/−, and IL-10−/− BALB/c mice, manually teased into small pieces, and incubated overnight and the supernatants analyzed by ELISA for IL-6 and IL-10. Cytokine levels were normalized to 1 g tumor tissue/mL media. Data are pooled from four independent experiments. Statistical significance was assessed by _t_-test.
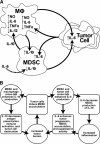
Figure 7.. Summary of cross-talk among MDSC, macrophages (MΦ), and tumor cells.
(A) Cross-talk with respect to IL-10, IL-6, IL-12, TNF-α, and NO. Solid arrows indicate direct effects mediated by the cell type or IL-10. Dashed arrow indicates an indirect effect by IL-6. (B) Potential cycle by IL-6, IL-10, and MDSC cross-talk promotes inflammation and tumor progression.
Similar articles
- Myeloid-derived suppressor cell function is reduced by Withaferin A, a potent and abundant component of Withania somnifera root extract.
Sinha P, Ostrand-Rosenberg S. Sinha P, et al. Cancer Immunol Immunother. 2013 Nov;62(11):1663-73. doi: 10.1007/s00262-013-1470-2. Epub 2013 Aug 27. Cancer Immunol Immunother. 2013. PMID: 23982485 Free PMC article. - Cross-talk between myeloid-derived suppressor cells and macrophages subverts tumor immunity toward a type 2 response.
Sinha P, Clements VK, Bunt SK, Albelda SM, Ostrand-Rosenberg S. Sinha P, et al. J Immunol. 2007 Jul 15;179(2):977-83. doi: 10.4049/jimmunol.179.2.977. J Immunol. 2007. PMID: 17617589 - Inflammation enhances myeloid-derived suppressor cell cross-talk by signaling through Toll-like receptor 4.
Bunt SK, Clements VK, Hanson EM, Sinha P, Ostrand-Rosenberg S. Bunt SK, et al. J Leukoc Biol. 2009 Jun;85(6):996-1004. doi: 10.1189/jlb.0708446. Epub 2009 Mar 4. J Leukoc Biol. 2009. PMID: 19261929 Free PMC article. - Cross-talk between myeloid-derived suppressor cells (MDSC), macrophages, and dendritic cells enhances tumor-induced immune suppression.
Ostrand-Rosenberg S, Sinha P, Beury DW, Clements VK. Ostrand-Rosenberg S, et al. Semin Cancer Biol. 2012 Aug;22(4):275-81. doi: 10.1016/j.semcancer.2012.01.011. Epub 2012 Feb 1. Semin Cancer Biol. 2012. PMID: 22313874 Free PMC article. Review. - The gastrointestinal tumor microenvironment.
Quante M, Varga J, Wang TC, Greten FR. Quante M, et al. Gastroenterology. 2013 Jul;145(1):63-78. doi: 10.1053/j.gastro.2013.03.052. Epub 2013 Apr 10. Gastroenterology. 2013. PMID: 23583733 Free PMC article. Review.
Cited by
- Cancer-Associated Myeloid Regulatory Cells.
De Vlaeminck Y, González-Rascón A, Goyvaerts C, Breckpot K. De Vlaeminck Y, et al. Front Immunol. 2016 Mar 29;7:113. doi: 10.3389/fimmu.2016.00113. eCollection 2016. Front Immunol. 2016. PMID: 27065074 Free PMC article. Review. - The effects of dendritic cell-based vaccines in the tumor microenvironment: Impact on myeloid-derived suppressor cells.
Sánchez-León ML, Jiménez-Cortegana C, Cabrera G, Vermeulen EM, de la Cruz-Merino L, Sánchez-Margalet V. Sánchez-León ML, et al. Front Immunol. 2022 Nov 15;13:1050484. doi: 10.3389/fimmu.2022.1050484. eCollection 2022. Front Immunol. 2022. PMID: 36458011 Free PMC article. Review. - Immunosuppression mediated by myeloid-derived suppressor cells (MDSCs) during tumour progression.
Groth C, Hu X, Weber R, Fleming V, Altevogt P, Utikal J, Umansky V. Groth C, et al. Br J Cancer. 2019 Jan;120(1):16-25. doi: 10.1038/s41416-018-0333-1. Epub 2018 Nov 9. Br J Cancer. 2019. PMID: 30413826 Free PMC article. Review. - Myeloidcells in the immunosuppressive microenvironment in glioblastoma: The characteristics and therapeutic strategies.
Huang B, Zhang J, Zong W, Chen S, Zong Z, Zeng X, Zhang H. Huang B, et al. Front Immunol. 2023 Feb 27;14:994698. doi: 10.3389/fimmu.2023.994698. eCollection 2023. Front Immunol. 2023. PMID: 36923402 Free PMC article. Review.
References
- Viola A., Sarukhan A., Bronte V., Molon B. (2012) The pros and cons of chemokines in tumor immunology. Trends Immunol. 33, 496–504. - PubMed
- Sica A., Larghi P., Mancino A., Rubino L., Porta C., Totaro M. G., Rimoldi M., Biswas S. K., Allavena P., Mantovani A. (2008) Macrophage polarization in tumour progression. Semin. Cancer Biol. 18, 349–355. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases