Mind the gap: glucocorticoids modulate hippocampal glutamate tone underlying individual differences in stress susceptibility - PubMed (original) (raw)
Mind the gap: glucocorticoids modulate hippocampal glutamate tone underlying individual differences in stress susceptibility
C Nasca et al. Mol Psychiatry. 2015 Jun.
Abstract
Why do some individuals succumb to stress and develop debilitating psychiatric disorders, whereas others adapt well in the face of adversity? There is a gap in understanding the neural bases of individual differences in the responses to environmental factors on brain development and functions. Here, using a novel approach for screening an inbred population of laboratory animals, we identified two subpopulations of mice: susceptible mice that show mood-related abnormalities compared with resilient mice, which cope better with stress. This approach combined with molecular and behavioral analyses, led us to recognize, in hippocampus, presynaptic mGlu2 receptors, which inhibit glutamate release, as a stress-sensitive marker of individual differences to stress-induced mood disorders. Indeed, genetic mGlu2 deletion in mice results in a more severe susceptibility to stress, mimicking the susceptible mouse sub-population. Furthermore, we describe an underlying mechanism by which glucocorticoids, acting via mineralocorticoid receptors (MRs), decrease resilience to stress via downregulation of mGlu2 receptors. We also provide a mechanistic link between MRs and an epigenetic control of the glutamatergic synapse that underlies susceptibility to stressful experiences. The approach and the epigenetic allostasis concept introduced here serve as a model for identifying individual differences based upon biomarkers and underlying mechanisms and also provide molecular features that may be useful in translation to human behavior and psychopathology.
Figures
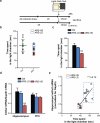
Figure 1. mGlu2 knockout mice show a severe susceptibility to stress
a and b, Time course and design of the chronic unpredictable stress (CUS). c, CUS results in a higher immobility time at the FST in mGlu2 knockout mice compared to wild-type mice (F3,72=64.02). Bars represent mean + SEM, * indicate significant comparisons to all other values, ***p < 0.0001. d and e, Body weight and scores at the coat-rating scale over the 4 weeks of CUS in wild-type mice and mGlu2 knock-out mice. Data represent mean + SEM, * indicate significant comparisons to corresponding controls, **p < 0.01, ***p < 0.0001.
Figure 2. Chronic unpredictable stress results in more (HS) and less (LS) susceptible endophenotypes and in individual difference in mGlu2 receptor expression: a molecular signature of susceptibility
a, Time course of the CUS and behavioral outcome analyses b, CUS results in a clear separation in HS and LS-endophenotypes at the coat-state rating scale for the evaluation of the CUS-induced coat deterioration (F14,375=47.19). c, Animal body weight over the four weeks of CUS (F14,375=206.5; F25,350=3.62). d and e, Identification of high and low-susceptible subgroups based on the immobility time at the sucrose intake (d) and at the forced swim test (e) in CUS-mice. Green lines indicate the mean (60.92 in d; 67.08 in e) and the standard deviation (in 15.20; 32.82 in c) of the control group. f, HS-mice show a lower sucrose intake at the sucrose preference test compared to LS wild-type mice subjected to CUS and unstressed mice (F5,66=11.61). g, HS-mice show a higher immobility time at the FST compared to LS wild-type mice subjected to CUS and unstressed mice (F5,74=40.67). Bars represent mean + SEM, * indicate significant comparisons to corresponding controls, ***p < 0.0001. h and i, Western blot analysis and representative blots of HS-mice show lower mGlu2 hippocampal receptor expression compared to CUS-LS mice and unstressed mice (F2,15=264.7). j and k, Western blot analysis and representative blots of mGlu2 receptor expression in the prefrontal cortex (F2,15=185.9) shows a strong impairment in both CUS-HS and CUS-LS mice compared to unstressed mice. Bars represent mean + SEM, * indicate significant comparisons to respective control groups, ***p < 0.0001.
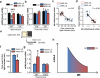
Figure 3. A single episode of stress results in more and less susceptible endophenotypes reminiscent of the CUS-induced HS and LS animal clusters
a, Time course and design of the acute stress and behavioral outcome. b, Identification of high and low-susceptible subgroups based on the time spent in the light chamber at the light dark test in acute restraint stressed-mice. Green lines indicate the mean (153.5) and the standard deviation (42.72) of the control group. c, Acute restraint stress results in behavioral and molecular individual differences that are reminiscent of the CUS HS and LS-endophenotypes. ARS-HS mice spent less time in the light chamber at the LDT compared to ARS-LS mice and unstressed mice (F2,27=10.75). d, ARS-HS mice show lower mGlu2 mRNA transcript levels compared to ARS-LS mice and unstressed mice within the hippocampus (F2,27=8.84) and no difference within the prefrontal cortex (F2,17=0.57). In a and c, bars represent mean + SEM, * indicate significant comparisons to all other values, **p < 0.01. e, Spearman's correlation analysis of hippocampal mGlu2 mRNA levels and time spent in the light chamber at the LDT in HS and LS-mice (r=0.75, p<0.0001).
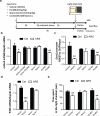
Figure 4. Glucocorticoids, via hippocampal down-regulation of MR receptors, lead to the loss of suppression of mGlu2 transcripts in resilient mice
a, Acute restraint stress results in lower MR mRNA transcript levels in LS mice compared to HS mice and unstressed mice within the hippocampus (F2,25=44.55) and no difference in GR levels (F2,12=1.32). b, ARS-LS mice compared to ARS-HS mice and unstressed mice show no difference in either MR or GR transcript levels within the PFC (MR: F2,17=2.13; GR:F2,13=0.13). In a and b, bars represent mean + SEM, * indicate significant comparisons to to all other values, **p < 0.01. c, Spearman's correlation analysis of hippocampal MR mRNA levels and time spent in the light chamber at the LDT in HS and LS-mice (r=-0.67, p=0.01). d, Spearman's correlation analysis of hippocampal MR mRNA levels and hippocampal mGlu2 mRNA levels (r=-0.98, p<0.0001). e, Design of the behavioral outcome on naïve mice. f, Naïve mice show behavioral differences in the time spent in the light chamber at the LDT. g, Naïve mice show different MR mRNA transcript levels based on their baseline susceptibility and no difference in mGlu2 mRNA transcript levels. In e and f, bars represent mean + SEM, * indicate significant comparisons, **p < 0.01, ***p < 0.001. h, In the hippocampus, the relationship between MR and mGlu2 mRNA levels can be described by a quadratic curve that models the epigenetic allostasis concept described in the text.
Figure 5. Glucocorticoids, via MR receptors, link stressful experience to an epigenetic control of the glutamatergic synapse
a, Time course and design of the treatments with the selective GR and MR antagonists. b, Spironolactone (20mg/kg, i.p.) alone or in combination with RU486 (also known as mifepristone, 20mg/kg, i.p.), but not RU486 alone, administered 3 hrs prior to the stress exposure, blocks the stress-induced decrease in mGlu2 mRNA transcript levels in the hippocampus of ARS mice c, Chromatin immunoprecipitation assay shows that spironolactone, but not RU486, blocks the stress-induced decrease in H3K27ac bound to the Grm2 promoter gene within the hippocampus. d, Spironolactone abolishes ARS reduction in the HAT p300 mRNA levels. e, Spironolactone, but not RU486, blocks the ARS-induced anxiety-related behavior at the light-dark test. Bars represent mean + SEM, * indicate significant comparisons to control groups, *p < 0.05, **p < 0.01, ***p <0.0001.
Similar articles
- Role of the Astroglial Glutamate Exchanger xCT in Ventral Hippocampus in Resilience to Stress.
Nasca C, Bigio B, Zelli D, de Angelis P, Lau T, Okamoto M, Soya H, Ni J, Brichta L, Greengard P, Neve RL, Lee FS, McEwen BS. Nasca C, et al. Neuron. 2017 Oct 11;96(2):402-413.e5. doi: 10.1016/j.neuron.2017.09.020. Neuron. 2017. PMID: 29024663 - Genetic, pharmacological and lesion analyses reveal a selective role for corticohippocampal GLUN2B in a novel repeated swim stress paradigm.
Kiselycznyk C, Svenningsson P, Delpire E, Holmes A. Kiselycznyk C, et al. Neuroscience. 2011 Oct 13;193:259-68. doi: 10.1016/j.neuroscience.2011.06.015. Epub 2011 Jun 23. Neuroscience. 2011. PMID: 21704131 Free PMC article. - Metabotropic glutamate receptor subtype 7 ablation causes dysregulation of the HPA axis and increases hippocampal BDNF protein levels: implications for stress-related psychiatric disorders.
Mitsukawa K, Mombereau C, Lötscher E, Uzunov DP, van der Putten H, Flor PJ, Cryan JF. Mitsukawa K, et al. Neuropsychopharmacology. 2006 Jun;31(6):1112-22. doi: 10.1038/sj.npp.1300926. Neuropsychopharmacology. 2006. PMID: 16237391 - Glutamate receptor antibodies in neurological diseases: anti-AMPA-GluR3 antibodies, anti-NMDA-NR1 antibodies, anti-NMDA-NR2A/B antibodies, anti-mGluR1 antibodies or anti-mGluR5 antibodies are present in subpopulations of patients with either: epilepsy, encephalitis, cerebellar ataxia, systemic lupus erythematosus (SLE) and neuropsychiatric SLE, Sjogren's syndrome, schizophrenia, mania or stroke. These autoimmune anti-glutamate receptor antibodies can bind neurons in few brain regions, activate glutamate receptors, decrease glutamate receptor's expression, impair glutamate-induced signaling and function, activate blood brain barrier endothelial cells, kill neurons, damage the brain, induce behavioral/psychiatric/cognitive abnormalities and ataxia in animal models, and can be removed or silenced in some patients by immunotherapy.
Levite M. Levite M. J Neural Transm (Vienna). 2014 Aug;121(8):1029-75. doi: 10.1007/s00702-014-1193-3. Epub 2014 Aug 1. J Neural Transm (Vienna). 2014. PMID: 25081016 Review. - Genomic and epigenomic mechanisms of glucocorticoids in the brain.
Gray JD, Kogan JF, Marrocco J, McEwen BS. Gray JD, et al. Nat Rev Endocrinol. 2017 Nov;13(11):661-673. doi: 10.1038/nrendo.2017.97. Epub 2017 Sep 1. Nat Rev Endocrinol. 2017. PMID: 28862266 Review.
Cited by
- Stress-induced structural plasticity of medial amygdala stellate neurons and rapid prevention by a candidate antidepressant.
Lau T, Bigio B, Zelli D, McEwen BS, Nasca C. Lau T, et al. Mol Psychiatry. 2017 Feb;22(2):227-234. doi: 10.1038/mp.2016.68. Epub 2016 May 31. Mol Psychiatry. 2017. PMID: 27240534 Free PMC article. - Bulk serum extracellular vesicles from stressed mice show a distinct proteome and induce behavioral and molecular changes in naive mice.
Monteleone MC, Billi SC, Abarzúa-Catalán L, Henzi R, Fernández EM, Kaehne T, Wyneken U, Brocco MA. Monteleone MC, et al. PLoS One. 2024 Aug 15;19(8):e0308976. doi: 10.1371/journal.pone.0308976. eCollection 2024. PLoS One. 2024. PMID: 39146369 Free PMC article. - BK channel deacetylation by SIRT1 in dentate gyrus regulates anxiety and response to stress.
Yu D, Homiack DR, Sawyer EJ, Schrader LA. Yu D, et al. Commun Biol. 2018 Jun 28;1:82. doi: 10.1038/s42003-018-0088-5. eCollection 2018. Commun Biol. 2018. PMID: 30271963 Free PMC article. - Cardiovascular outcomes related to social defeat stress: New insights from resilient and susceptible rats.
Morais-Silva G, Costa-Ferreira W, Gomes-de-Souza L, Pavan JC, Crestani CC, Marin MT. Morais-Silva G, et al. Neurobiol Stress. 2019 Jun 6;11:100181. doi: 10.1016/j.ynstr.2019.100181. eCollection 2019 Nov. Neurobiol Stress. 2019. PMID: 31236438 Free PMC article. - Meet Our Regional Editor.
Nicoletti F. Nicoletti F. Curr Neuropharmacol. 2018 Feb;16(2):117. doi: 10.2174/1570159X1602180130155228. Curr Neuropharmacol. 2018. PMID: 29681788 Free PMC article. No abstract available.
References
- Freund J, Brandmaier AM, Lewejohann L, Kirste I, Kritzlerm M, Kruger A. Emergence of individuality in genetically identical mice. Science. 2013;340:756–9. - PubMed
- Miller MM, Morrison JH, McEwen BS. Basal anxiety-like behavior predicts differences in dendritic morphology in the medial prefrontal cortex in two strains of rats. Behav Brain Res. 2012;229:280–8. - PubMed
- Southwick SM, Charney DS. The Science of Resilience: Implications for the Prevention and Treatment of Depression. Science. 2012;338:79–82. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous