Loss of PIKfyve in platelets causes a lysosomal disease leading to inflammation and thrombosis in mice - PubMed (original) (raw)
Aae Suzuki 1, Timothy J Stalker 1, Liang Zhao 1, Yuhuan Wang 2, Chris McKennan 3, Matthew J Riese 1, Jessica F Guzman 1, Suhong Zhang 4, Lurong Lian 1, Rohan Joshi 1, Ronghua Meng 5, Steven H Seeholzer 3, John K Choi 6, Gary Koretzky 1, Michael S Marks 5, Charles S Abrams 1
Affiliations
- PMID: 25178411
- PMCID: PMC4369914
- DOI: 10.1038/ncomms5691
Loss of PIKfyve in platelets causes a lysosomal disease leading to inflammation and thrombosis in mice
Sang H Min et al. Nat Commun. 2014.
Abstract
PIKfyve is essential for the synthesis of phosphatidylinositol-3,5-bisphosphate [PtdIns(3,5)P2] and for the regulation of endolysosomal membrane dynamics in mammals. PtdIns(3,5)P2 deficiency causes neurodegeneration in mice and humans, but the role of PtdIns(3,5)P2 in non-neural tissues is poorly understood. Here we show that platelet-specific ablation of PIKfyve in mice leads to accelerated arterial thrombosis, and, unexpectedly, also to inappropriate inflammatory responses characterized by macrophage accumulation in multiple tissues. These multiorgan defects are attenuated by platelet depletion in vivo, confirming that they reflect a platelet-specific process. PIKfyve ablation in platelets induces defective maturation and excessive storage of lysosomal enzymes that are released upon platelet activation. Impairing lysosome secretion from PIKfyve-null platelets in vivo markedly attenuates the multiorgan defects, suggesting that platelet lysosome secretion contributes to pathogenesis. Our findings identify PIKfyve as an essential regulator for platelet lysosome homeostasis, and demonstrate the contributions of platelet lysosomes to inflammation, arterial thrombosis and macrophage biology.
Figures
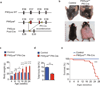
Figure 1. Platelet-specific ablation of PIKfyve causes multiorgan abnormalities in mice
(a) Schematic representation of genetic targeting of PIKfyve. The target exons 37and 38 (red bars) were targeted with lox P recombination sites (yellow arrows) to generate PIKfyve floxed alleles (PIKfyvefl). Mice expressing PIKfyvefl were then crossed with Pf4-Cre mice to induce homologous recombination of the PIKfyvefl (PIKfyve Post-Cre). (b) General appearance of control and PIKfyvefl/fl Pf4-Cre littermates at 24 weeks of age. Note the characteristic coarse facial features, body hair loss, and severely distended body morphology. (c) Body weight of control mice and PIKfyvefl/fl Pf4-Cre mice. The average numbers of mice were 8 per time point and per group. (d) Percent of total body fat in the control (n=3) and PIKfyvefl/fl Pf4-Cre (n=3) mice at 12–24 weeks of age. (e) Survival curves of control (n= 40) and PIKfyvefl/fl Pf4-Cre (n= 40) mice. *P<0.05, ** P<0.01, ***P<0.001. All error bars indicate mean +/− S.D.
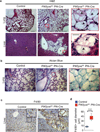
Figure 2. Large vacuolated macrophages infiltrate multiple tissues in the PIKfyve fl/fl Pf4-Cre mice
(a) Representative histological sections of the lungs and livers stained with hematoxylin and eosin from control mice and PIKfyvefl/fl Pf4-Cre mice. Scale bar, 100µm. Insets show increased magnification of boxed areas in panel (a). Scale bar, 20µm. Arrowheads indicate the vacuolated cells. (b) Lung sections stained with Alcian blue. Scale bar, 100µm. Inset shows high magnification of the boxed area. (c) Liver structures of control mice and PIKfyvefl/fl Pf4-Cre mice were stained for macrophages with anti-F4/80 by immunohistochemistry. Scale bar, 100µm. Insets in the upper right corner of each image show higher magnification images of boxed areas. Scale bar, 20µm. (d) Cell diameters of F4/80+ cells in the livers of control mice (n=5) and PIKfyvefl/fl Pf4-Cre mice (n=5). ***P<0.001. All error bars indicate mean +/− S.D.
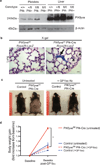
Figure 3. Platelets drive the multiorgan defects in the PIKfyvefl/fl Pf4-Cre mice
(a) The levels of PIKfyve protein were determined in the platelets and liver of the indicated mice by immunoblotting. Note the absence of the PIKfyve protein in the platelets, but its presence in the liver of PIKfyvefl/fl Pf4-Cre mice (n=3). Tissues from the wild-type mice, the PIKfyvefl/+ Pf4-Cre mice, and the PIKfyvefl/fl mice were used as positive controls. β-actin was used as a loading control. (b) X-gal staining of the lung tissues from PIKfyvefl/fl Rosa26-LacZ mice and PIKfyvefl/fl Pf4-Cre Rosa26-LacZ mice. Scale bar, 20µm. The arrow indicates the platelet clumps in the lung vessels expressing LacZ (blue stain). Arrowheads indicate the vacuolated macrophages that did not stain blue. (c) Representative general morphology of 15-weeks-old control mice and PIKfyvefl/fl Pf4-Cre mice after 6 weeks of either no intervention (untreated) or biweekly injections of anti-GP1bα antibodies (+GP1bα Ab). (d) Body weight change of the control mice and PIKfyvefl/fl Pf4-Cre mice after 6 weeks of no intervention or treatment with anti-GP1bα antibodies (n=3 for each group). *P<0.05. All error bars indicate mean +/− S.D.
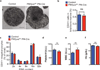
Figure 4. PIKfyve is not necessary for the development of megakaryocytes nor for platelet production
(a) Electron micrographs of megakaryocytes grown in culture in the presence of thrombopoietin for 7 days. Note multiple enlarged vacuoles in the cytoplasm of PIKfyvefl/fl Pf4-Cre mice as compared to the control mice. Scale bar, 10µm. (b) CD41+ megakaryocyte counts of control PIKfyvefl/fl Pf4-Cre mice. (c) Megakaryocyte ploidy. (d–f) Counts of platelets (d), WBC (e), and Hb (f) of control mice and PIKfyvefl/fl Pf4-Cre mice.
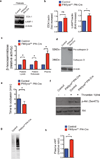
Figure 5. PIKfyve is critical for the maturation, storage, and release of lysosomal enzymes in platelets
(a) Immunoblot of platelet lysates from the control mice and PIKfyvefl/fl Pf4-Cre mice showing the expression of EEA-1 and LAMP-1. β-actin was used as the loading control. (b) Quantification of protein bands shown in the panel a (n=3 per group). (c) β-hexosaminidase activity in the platelet lysates of control mice (n=3) and PIKfyvefl/fl Pf4-Cre mice (n=3). (d) β-hexosaminidase activity in the platelet releasate and plasma of control mice (n=3) and PIKfyvefl/fl Pf4-Cre mice (n=3). (e) Time to occlusion (TTO) of carotid arteries upon vascular injury with 7.5% of FeCl3 in the control mice (n=9) and the PIKfyvefl/fl Pf4-Cre mice (n=8 ). (f) Immunoblot of platelet lysates from control platelets and PIKfyvefl/fl Pf4-Cre platelets showing the Akt phosphorylation in resting condition or stimulated with 1U/mL of thrombin for 10 minutes. (g) SDS agarose electrophoresis showing the multimer pattern of plasma vWF from the control mice (n=3) and the PIKfyvefl/fl Pf4-Cre (n=3) mice. (h) Plasma levels of vWF from the control mice (n=3) and the PIKfyvefl/fl Pf4-Cre (n=3) mice were quantified by ELISA. *P<0.05. All error bars indicate mean +/− S.D.
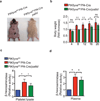
Figure 6. Impaired secretion of platelet lysosomes attenuates the phenotypes of PIKfyvefl/fl Pf4-Cre mice
(a) General appearance of PIKfyvefl/fl Pf4-Cre mice and PIKfyvefl/fl Pf4-Cre/pallid mice on C57BL/6 background at 17 weeks of age. In the presence of the pallid mutation, the hair loss and weight gain are attenuated in the PIKfyvefl/fl Pf4-Cre/pallid mice. Note that the pallid mice have an underlying defect in melanosome biogenesis causing hypopigmented fur. (b) Body weight of the PIKfyvefl/fl Pf4-Cre mice and the aged-matched PIKfyve Pf4-Cre/pallid mice. The average numbers of mice were 5 per time point and per group. The dashed line indicates the mean ratio of body weight of the control mice. (c,d) Analysis of β-hexosaminidase activity in the platelet lysate (c) and plasma (d) of the PIKfyvefl/fl Pf4-Cre mice (n=3) and the PIKfyvefl/fl Pf4-Cre/pallid (n=3) mice. *P <0.05, ** P < 0.01. All error bars indicate mean +/− S.D.
Similar articles
- PIKfyve Deficiency in Myeloid Cells Impairs Lysosomal Homeostasis in Macrophages and Promotes Systemic Inflammation in Mice.
Min SH, Suzuki A, Weaver L, Guzman J, Chung Y, Jin H, Gonzalez F, Trasorras C, Zhao L, Spruce LA, Seeholzer SH, Behrens EM, Abrams CS. Min SH, et al. Mol Cell Biol. 2019 Oct 11;39(21):e00158-19. doi: 10.1128/MCB.00158-19. Print 2019 Nov 1. Mol Cell Biol. 2019. PMID: 31427458 Free PMC article. - PIKfyve-Dependent Phosphoinositide Dynamics in Megakaryocyte/Platelet Granule Integrity and Platelet Functions.
Caux M, Mansour R, Xuereb JM, Chicanne G, Viaud J, Vauclard A, Boal F, Payrastre B, Tronchère H, Severin S. Caux M, et al. Arterioscler Thromb Vasc Biol. 2022 Aug;42(8):987-1004. doi: 10.1161/ATVBAHA.122.317559. Epub 2022 Jun 16. Arterioscler Thromb Vasc Biol. 2022. PMID: 35708031 - Core protein machinery for mammalian phosphatidylinositol 3,5-bisphosphate synthesis and turnover that regulates the progression of endosomal transport. Novel Sac phosphatase joins the ArPIKfyve-PIKfyve complex.
Sbrissa D, Ikonomov OC, Fu Z, Ijuin T, Gruenberg J, Takenawa T, Shisheva A. Sbrissa D, et al. J Biol Chem. 2007 Aug 17;282(33):23878-91. doi: 10.1074/jbc.M611678200. Epub 2007 Jun 7. J Biol Chem. 2007. PMID: 17556371 - Phosphatidylinositol 3 monophosphate metabolizing enzymes in blood platelet production and in thrombosis.
Bellio M, Caux M, Vauclard A, Chicanne G, Gratacap MP, Terrisse AD, Severin S, Payrastre B. Bellio M, et al. Adv Biol Regul. 2020 Jan;75:100664. doi: 10.1016/j.jbior.2019.100664. Epub 2019 Oct 7. Adv Biol Regul. 2020. PMID: 31604685 Review. - Phosphoinositide 3-kinases in platelets, thrombosis and therapeutics.
Ribes A, Oprescu A, Viaud J, Hnia K, Chicanne G, Xuereb JM, Severin S, Gratacap MP, Payrastre B. Ribes A, et al. Biochem J. 2020 Nov 27;477(22):4327-4342. doi: 10.1042/BCJ20190402. Biochem J. 2020. PMID: 33242335 Review.
Cited by
- PIKfyve regulates melanosome biogenesis.
Liggins MC, Flesher JL, Jahid S, Vasudeva P, Eby V, Takasuga S, Sasaki J, Sasaki T, Boissy RE, Ganesan AK. Liggins MC, et al. PLoS Genet. 2018 Mar 27;14(3):e1007290. doi: 10.1371/journal.pgen.1007290. eCollection 2018 Mar. PLoS Genet. 2018. PMID: 29584722 Free PMC article. - PIKfyve/Fab1 is required for efficient V-ATPase and hydrolase delivery to phagosomes, phagosomal killing, and restriction of Legionella infection.
Buckley CM, Heath VL, Guého A, Bosmani C, Knobloch P, Sikakana P, Personnic N, Dove SK, Michell RH, Meier R, Hilbi H, Soldati T, Insall RH, King JS. Buckley CM, et al. PLoS Pathog. 2019 Feb 7;15(2):e1007551. doi: 10.1371/journal.ppat.1007551. eCollection 2019 Feb. PLoS Pathog. 2019. PMID: 30730983 Free PMC article. - Biallelic Mutations of VAC14 in Pediatric-Onset Neurological Disease.
Lenk GM, Szymanska K, Debska-Vielhaber G, Rydzanicz M, Walczak A, Bekiesinska-Figatowska M, Vielhaber S, Hallmann K, Stawinski P, Buehring S, Hsu DA, Kunz WS, Meisler MH, Ploski R. Lenk GM, et al. Am J Hum Genet. 2016 Jul 7;99(1):188-94. doi: 10.1016/j.ajhg.2016.05.008. Epub 2016 Jun 9. Am J Hum Genet. 2016. PMID: 27292112 Free PMC article. - The Phosphoinositide Kinase PIKfyve Promotes Cathepsin-S-Mediated Major Histocompatibility Complex Class II Antigen Presentation.
Baranov MV, Bianchi F, Schirmacher A, van Aart MAC, Maassen S, Muntjewerff EM, Dingjan I, Ter Beest M, Verdoes M, Keyser SGL, Bertozzi CR, Diederichsen U, van den Bogaart G. Baranov MV, et al. iScience. 2019 Jan 25;11:160-177. doi: 10.1016/j.isci.2018.12.015. Epub 2018 Dec 20. iScience. 2019. PMID: 30612035 Free PMC article. - Inhibition of PIKfyve Leads to Lysosomal Disorders via Dysregulation of mTOR Signaling.
Xia J, Wang H, Zhong Z, Jiang J. Xia J, et al. Cells. 2024 May 30;13(11):953. doi: 10.3390/cells13110953. Cells. 2024. PMID: 38891085 Free PMC article.
References
- Di Paolo G, De Camilli P. Phosphoinositides in cell regulation and membrane dynamics. Nature. 2006;443:651–657. - PubMed
- Sbrissa D, et al. Core protein machinery for mammalian phosphatidylinositol 3,5-bisphosphate synthesis turnover that regulates the progression of endosomal transport Novel Sac phosphatase joins the ArPIKfyve-PIKfyve complex. The Journal of biological chemistry. 2007;282:23878–23891. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32 HL07439/HL/NHLBI NIH HHS/United States
- P30 DK050306/DK/NIDDK NIH HHS/United States
- DK050306/DK/NIDDK NIH HHS/United States
- P01 HL040387/HL/NHLBI NIH HHS/United States
- R01 HL121323/HL/NHLBI NIH HHS/United States
- R01 HL110110/HL/NHLBI NIH HHS/United States
- HL110110/HL/NHLBI NIH HHS/United States
- HL40387/HL/NHLBI NIH HHS/United States
- HL120846/HL/NHLBI NIH HHS/United States
- P01 HL120846/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases