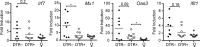Early, transient depletion of plasmacytoid dendritic cells ameliorates autoimmunity in a lupus model - PubMed (original) (raw)
Early, transient depletion of plasmacytoid dendritic cells ameliorates autoimmunity in a lupus model
Sarah L Rowland et al. J Exp Med. 2014.
Abstract
Plasmacytoid dendritic cells (pDCs) have long been implicated in the pathogenesis of lupus. However, this conclusion has been largely based on a correlative link between the copious production of IFN-α/β by pDCs and the IFN-α/β "signature" often seen in human lupus patients. The specific contribution of pDCs to disease in vivo has not been investigated in detail. For this reason, we generated a strain of BXSB lupus-prone mice in which pDCs can be selectively depleted in vivo. Early, transient ablation of pDCs before disease initiation resulted in reduced splenomegaly and lymphadenopathy, impaired expansion and activation of T and B cells, reduced antibodies against nuclear autoantigens and improved kidney pathology. Amelioration of pathology coincided with decreased transcription of IFN-α/β-induced genes in tissues. PDC depletion had an immediate impact on the activation of immune cells, and importantly, the beneficial effects on pathology were sustained even though pDCs later recovered, indicating an early pDC contribution to disease. Together, our findings demonstrate a critical function for pDCs during the IFN-α/β-dependent initiation of autoimmune lupus and point to pDCs as an attractive therapeutic target for the treatment of SLE.
© 2014 Rowland et al.
Figures
Figure 1.
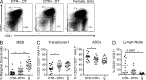
Reduced lymphoid organ hyperplasia in pDC-depleted BXSB.DTR mice. (A) Representative flow cytometric analysis of B220+SiglecH+ pDCs in spleen of 11-wk-old BXSB.DTR male littermates that were treated with DT as indicated. Female littermates were untreated (Untx). Spleen weight (B) and absolute spleen cell number (C) were determined in indicated mice. Number of CD19+ splenocytes (D), CD3+ splenocytes (E), and CD4+ splenic T cells (F) was determined by flow cytometry. (G) Frequency and absolute cell number of splenic pDCs and CD86 expression on splenic pDCs (H) was assessed by flow cytometry and CD86 expression is represented as geometric MFI. In B–H, male DTR+ and DTR– mice were treated with DT and analyzed at 19 wk of age. Untreated, healthy female littermates are shown. Data points indicate individual mice grouped from 10–11 experiments. Bar indicates arithmetic mean. *, P < 0.5; **, P < 0.01; ***, P < 0.001. Absence of * indicates nonsignificant differences.
Figure 2.
Transient pDC ablation impairs expansion and activation of T cells in BXSB.DTR mice. Male DTR+ and DTR– mice were treated with DT and analyzed at 19 wk of age. Untreated, healthy female littermates are shown. (A) Representative flow cytometric analysis of CD44 and CD62L expression on CD4+CD3+ splenocytes in the indicated groups of mice. Frequency of CD44highCD62Llow (B), CD44low/–CD62L+ (C), and CD69+ CD4+ and CD69+CD8+ (D) T cells in spleen of the indicated mice were determined by flow cytometry. Data points indicate individual mice grouped from 10–11 experiments. Bar indicates arithmetic mean. *, P < 0.5; **, P < 0.01; ***, P < 0.001. Absence of * indicates nonsignificant differences.
Figure 3.
Transient pDC depletion reconstitutes MZ B cells and inhibits the development of ABCs. Male DTR+ and DTR– mice were treated with DT and analyzed at 19 wk of age. Untreated, healthy female littermates are shown. (A) Representative FACS analysis of CD23 and CD21 expression on CD19+ splenocytes. Frequencies of CD23–CD21high MZ B cells (B) and CD23–CD21–AA4.1+ transitional-1 B cells and CD23–CD21–AA4.1– ABCs (C) in spleen of indicated mice were determined by flow cytometry. Frequencies of CD23–CD21– CD19+ cells in lymph node (D) of indicated mice were determined by flow cytometry. Data points indicate individual mice grouped from 10–11 experiments. Bar indicates arithmetic mean. *, P < 0.5; **, P < 0.01; ***, P < 0.001. Absence of * indicates nonsignificant differences.
Figure 4.
Antinuclear antibodies are reduced in BXSB.DTR mice after transient pDC depletion. Male DTR+ and DTR– mice were treated with DT and analyzed at 19 wk of age. Untreated, healthy female littermates are shown. (A) Total IgG concentration in serum of indicated mice. (B) Antinuclear antibodies in sera were analyzed by HEp-2 assay. (left) Representative images are shown; (right) serum ANAs were titered to endpoint and Mann-Whitney test was applied. N.D. indicates signal was not detected at 1:40 dilution. (C) Concentration of IgG isotypes in serum of indicated mice was determined by ELISA. (D) Mean total serum immunoglobulin concentrations in indicated mice. (E) Sera from 19-wk-old male mice were subjected to an autoantigen microarray. Heat map of IgG antibody reactivity to indicated autoantigens in pDC-sufficient (BXSB.DTR–, DT) and pDC-depleted (BXSB.DTR+, DT) mice is shown. Each column represents an individual mouse; each row indicates a single antigen. Relative maximum row signals are shown in red; relative minimum row signals are shown in blue. (F) Normalized signal of serum IgG reactivity for indicated antigens, as analyzed by autoantigen microarray described in (E). Data points indicate individual mice grouped from 10 experiments. Bar indicates arithmetic mean. *, P < 0.5; **, P < 0.01; ***, P < 0.001. Absence of * indicates nonsignificant differences.
Figure 5.
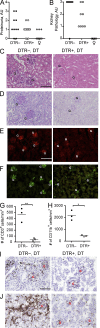
Early pDC depletion reduces severity of kidney pathology in BXSB.DTR mice. Male DTR+ and DTR– mice were treated with DT and analyzed at 19 wk of age. Untreated, healthy female littermates are shown. Proteinuria (A) and glomerulonephritis (B) were assessed in indicated mice. Formalin-fixed kidney sections were stained with H&E (C) or PAS (D). Images are representative of four to eight animals per group. Frozen kidney sections were stained for IgG (E, red) and C3 (F, green) to identify immune complexes. Relative abundance of infiltrating CD3+ (G) and CD11b+ (H) cells in kidney of indicated mice were calculated. Images are representative of three animals per group. Frozen kidney sections were stained for (I) CD3 and (J) CD11b expression. Images are representative of three animals per group. Individual glomeruli are marked in histology panels with the letter G. The magnification in C–F and I–J is 200×. Bars: (C, E, and I) 100 µm. Data points indicate individual mice from three experiments. *, P < 0.5; **, P < 0.01; ***, P < 0.001. Absence of * indicates nonsignificant differences.
Figure 6.
Local expression of IFN-α/β–stimulated genes in kidney tissues is reduced after pDC depletion. Male DTR+ and DTR– mice were treated with DT and analyzed at 19 wk of age. Untreated, healthy female littermates are shown. Relative expression of indicated genes in kidney tissue was analyzed by RT-PCR. Data points indicate individual mice grouped from eight experiments. Bar indicates mean relative expression of indicated genes. *, P < 0.5; **, P < 0.01; ***, P < 0.001. Absence of * indicates nonsignificant differences.
Figure 7.
Transient pDC depletion has an immediate effect on the activation and expansion of innate and adaptive immune cells. Male mice were treated with DT and analyzed at 11 wk of age. Untreated, healthy female littermates are shown. (A) Frequencies of B220+SiglecH+ pDCs 24 h after last DT treatment were determined by flow cytometry. (B) Spleen weight and absolute cell number were calculated in indicated mice. (C) Number of CD4+ and CD19+ splenocytes. Frequencies of CD44highCD62Llow (D), CD44low/–CD62L+ (E), and CD69+ CD4+ and CD69+CD8+ (F) T cells in spleen of indicated mice. (G) Frequencies of CD86+CD19+ splenocytes. (H) Number of CD11blow/–CD11chigh and CD11b+CD11chigh MHCII+ DCs in spleen of indicated mice. (I) Relative expression of indicated IFN-α/β–induced genes in peripheral blood was analyzed by RT-PCR. Data points indicate individual mice. Bar indicates arithmetic mean. *, P < 0.5; **, P < 0.01; ***, P < 0.001. Absence of * indicates nonsignificant differences.
Figure 8.
Transient pDC depletion has an immediate effect on the presence of circulating autoantibodies. Male DTR+ and DTR– mice were treated with DT and analyzed at 11 wk of age. Untreated, healthy female littermates are shown. (A–C) Total IgG concentration in serum (A), individual IgG isotypes (B), and mean total serum IgG concentration (C) was determined by ELISA in the indicated mice. (D) Sera from 11-wk-old male mice were subjected to an autoantigen microarray. Heat map of IgG antibody reactivity to indicated autoantigens in pDC-sufficient (BXSB.DTR–, DT) and pDC-depleted (BXSB.DTR+, DT) mice. Each column represents an individual mouse; each row indicates a single antigen. Relative maximum row signals are shown in red; relative minimum row signals are shown in blue. (E) Normalized signal of serum IgG reactivity for indicated antigens, as analyzed by autoantigen microarray described in (D). Serum autoantibodies (IgG) were analyzed by autoantigen microarray as in (D) and normalized signals for the indicated antigens are shown. Data points represent individual mice. Bar indicates arithmetic mean. *, P < 0.5; **, P < 0.01; ***, P < 0.001. Absence of * indicates nonsignificant differences.
Figure 9.
Reduced lupus-associated soluble factors and inflammatory chemokines in sera after early pDC depletion. Male DTR+ and DTR– mice were treated with DT and analyzed at 11 or 19 wk of age, as marked. Age-matched, untreated, healthy female littermates are included. Concentration of soluble (A) VCAM-1, (B) CD40L, (C) MPO, and (D) select chemokines in sera of indicated mice were determined by serum protein array. Data points indicate samples from individual mice analyzed by MyriadRBM rodent MAPv3.0 array. *, P < 0.5; **, P < 0.01; ***, P < 0.001. Absence of * indicates nonsignificant differences.
Comment in
- Connective tissue diseases: depleting plasmacytoid dendritic cells: a new therapeutic approach in SLE?
Onuora S. Onuora S. Nat Rev Rheumatol. 2014 Oct;10(10):573. doi: 10.1038/nrrheum.2014.160. Epub 2014 Sep 16. Nat Rev Rheumatol. 2014. PMID: 25224304 No abstract available.
Similar articles
- Bcl-2 antagonists kill plasmacytoid dendritic cells from lupus-prone mice and dampen interferon-α production.
Zhan Y, Carrington EM, Ko HJ, Vikstrom IB, Oon S, Zhang JG, Vremec D, Brady JL, Bouillet P, Wu L, Huang DC, Wicks IP, Morand EF, Strasser A, Lew AM. Zhan Y, et al. Arthritis Rheumatol. 2015 Mar;67(3):797-808. doi: 10.1002/art.38966. Arthritis Rheumatol. 2015. PMID: 25418983 - Sialic acid-binding immunoglobulin-type lectin H-positive plasmacytoid dendritic cells drive spontaneous lupus-like disease development in B6.Nba2 mice.
Davison LM, Jørgensen TN. Davison LM, et al. Arthritis Rheumatol. 2015 Apr;67(4):1012-22. doi: 10.1002/art.38989. Arthritis Rheumatol. 2015. PMID: 25504931 - Genetic evidence for the role of plasmacytoid dendritic cells in systemic lupus erythematosus.
Sisirak V, Ganguly D, Lewis KL, Couillault C, Tanaka L, Bolland S, D'Agati V, Elkon KB, Reizis B. Sisirak V, et al. J Exp Med. 2014 Sep 22;211(10):1969-76. doi: 10.1084/jem.20132522. Epub 2014 Sep 1. J Exp Med. 2014. PMID: 25180061 Free PMC article. - Predominant Role of Plasmacytoid Dendritic Cells in Stimulating Systemic Autoimmunity.
Huang X, Dorta-Estremera S, Yao Y, Shen N, Cao W. Huang X, et al. Front Immunol. 2015 Oct 12;6:526. doi: 10.3389/fimmu.2015.00526. eCollection 2015. Front Immunol. 2015. PMID: 26528288 Free PMC article. Review. - A Plasmacytoid Dendritic Cells-Type I Interferon Axis Is Critically Implicated in the Pathogenesis of Systemic Lupus Erythematosus.
Kim JM, Park SH, Kim HY, Kwok SK. Kim JM, et al. Int J Mol Sci. 2015 Jun 23;16(6):14158-70. doi: 10.3390/ijms160614158. Int J Mol Sci. 2015. PMID: 26110387 Free PMC article. Review.
Cited by
- CAR-T cells and CAR-Tregs targeting conventional type-1 dendritic cell suppress experimental autoimmune encephalomyelitis.
Moorman CD, Yu S, Briseno CG, Phee H, Sahoo A, Ramrakhiani A, Chaudhry A. Moorman CD, et al. Front Immunol. 2023 Oct 27;14:1235222. doi: 10.3389/fimmu.2023.1235222. eCollection 2023. Front Immunol. 2023. PMID: 37965348 Free PMC article. - STING Mediates Lupus via the Activation of Conventional Dendritic Cell Maturation and Plasmacytoid Dendritic Cell Differentiation.
Thim-Uam A, Prabakaran T, Tansakul M, Makjaroen J, Wongkongkathep P, Chantaravisoot N, Saethang T, Leelahavanichkul A, Benjachat T, Paludan S, Pisitkun T, Pisitkun P. Thim-Uam A, et al. iScience. 2020 Sep 4;23(9):101530. doi: 10.1016/j.isci.2020.101530. eCollection 2020 Sep 25. iScience. 2020. PMID: 33083760 Free PMC article. - Lymph Node Stromal Cells: Mapmakers of T Cell Immunity.
Harlé G, Kowalski C, Garnier L, Hugues S. Harlé G, et al. Int J Mol Sci. 2020 Oct 21;21(20):7785. doi: 10.3390/ijms21207785. Int J Mol Sci. 2020. PMID: 33096748 Free PMC article. Review. - Functional Characterization of CD11c+ Age-Associated B Cells as Memory B Cells.
Du SW, Arkatkar T, Al Qureshah F, Jacobs HM, Thouvenel CD, Chiang K, Largent AD, Li QZ, Hou B, Rawlings DJ, Jackson SW. Du SW, et al. J Immunol. 2019 Dec 1;203(11):2817-2826. doi: 10.4049/jimmunol.1900404. Epub 2019 Oct 21. J Immunol. 2019. PMID: 31636237 Free PMC article. - Female predisposition to TLR7-driven autoimmunity: gene dosage and the escape from X chromosome inactivation.
Souyris M, Mejía JE, Chaumeil J, Guéry JC. Souyris M, et al. Semin Immunopathol. 2019 Mar;41(2):153-164. doi: 10.1007/s00281-018-0712-y. Epub 2018 Oct 1. Semin Immunopathol. 2019. PMID: 30276444 Review.
References
- Baccala, R., Gonzalez-Quintial R., Blasius A.L., Rimann I., Ozato K., Kono D.H., Beutler B., and Theofilopoulos A.N.. 2013. Essential requirement for IRF8 and SLC15A4 implicates plasmacytoid dendritic cells in the pathogenesis of lupus. Proc. Natl. Acad. Sci. USA. 110:2940–2945 10.1073/pnas.1222798110 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases