TREM2 Protein Expression Changes Correlate with Alzheimer's Disease Neurodegenerative Pathologies in Post-Mortem Temporal Cortices - PubMed (original) (raw)
TREM2 Protein Expression Changes Correlate with Alzheimer's Disease Neurodegenerative Pathologies in Post-Mortem Temporal Cortices
Lih-Fen Lue et al. Brain Pathol. 2015 Jul.
Abstract
Triggering receptor expressed by myeloid cells 2 (TREM2), a member of the immunoglobulin superfamily, has anti-inflammatory phagocytic function in myeloid cells. Several studies have shown that TREM2 gene variant rs75932628-T increased the risks for Alzheimer's disease (AD), Parkinson's disease, frontotemporal dementia and amyotrophic lateral sclerosis. It has been suggested that the risks could be resulted from the loss of TREM2 function caused by the mutation. Indeed, new evidence showed that several mutations in the immunoglobulin-like V-region led to low cell surface expression of TREM2 and reduced phagocytic function. Because of the emerging importance in understanding TREM2 expression and functions in human neurodegenerative diseases, we conducted biochemical and morphological studies of TREM2 expression in human post-mortem temporal cortical samples from AD and normal cases. Increased expression of TREM2 protein was found to significantly correlate with increases of phosphorylated-tau and active caspase 3, a marker of apoptosis, and also loss of the presynaptic protein SNAP25. Strong intensities of TREM2 immunoreactivity were observed in the microglia associated with amyloid plaques and in neuritic pathology-enriched areas. Based on the findings that TREM2 expression correlated with neurodegenerative markers, further investigation on whether there is abnormality of TREM2 functions in AD brains with nonmutated TREM2 is needed.
Keywords: TREM2; amyloid; brain; human; microglia; tau.
© 2014 The Authors. Brain Pathology published by John Wiley & Sons Ltd on behalf of International Society of Neuropathology.
Figures
Figure 1
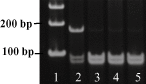
Identification of
TREM
2 (triggering receptor expressed by myeloid cells 2) rs75932628‐
T
.
DNA
samples from cerebellum were amplified by polymerase chain reaction (
PCR
) followed by restriction enzyme digestion using primer pairs that allow the identification of rs75932628 genotypes. The gel image of amplified
PCR
products is shown here. Lane 1 is the
DNA
ladder. In lane 2, the band at 172 bp indicated the presence of rs75932628‐
T
in this case caused by the loss of a
H
ha
I
restriction enzyme site. The other lanes were from
DNA
samples without this polymorphism. For the purpose of this study, we only used autopsy cases that did not have rs75932628‐
T
genotype.
Figure 2
Antibody characterization in the
HEK
cells and human brain homogenates. A. Lysate samples of
HEK
cells transfected with human
TREM
2 (triggering receptor expressed by myeloid cells 2) and
DAP
12 (DNAX‐activation protein 12) plasmids were used to characterize
TREM
2 antibodies by immunoblotting.
TREM
2 protein bands were detected in the range of molecular weights of 35–40 kDa with three antibodies, only in the samples with
TREM
2 transfection. Among these antibodies, the goat polyclonal antibody from
R
&
D
System performed more robustly in
TREM
2‐transfected cells.
DAP
12 protein bands were detected in
HEK
cells transfected with human
DAP
12 plasmids at 12 kDa, but not in the
TREM
2‐transfected samples.
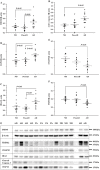
Figure 3
The expression levels of
TREM
2 (triggering receptor expressed by myeloid cells 2),
DAP
12 (DNAX‐activation protein 12),
IBA
1, cleaved caspase 3,
SNAP
25 and
PSD
95 by disease groups. The expression levels of microglial proteins
TREM
2,
DAP
12 and
IBA
1, apoptotic marker active caspase 3, and synaptic proteins
SANP
25 and
PSD
95 were analyzed in the brain samples using protein‐specific antibodies by our standard immunoblotting procedure. The intensities of the specific band in each sample were normalized by β‐actin followed by statistical analysis by disease groups: nondemented controls (
ND
); possible
AD
(
P
oss
AD
); and
A
lzheimer's disease (
AD
). Significant changes (P < 0.05) were detected between
AD
and
ND
groups in
TREM
2 (A),
DAP
12 (B),
IBA
1 (C), and active caspase 3 (D),
SNAP
25 (E) and
PSD
95 (F). However, the significant differences between
AD
and
P
oss
AD
groups were only detected in
TREM
2,
DAP
12 and
IBA
1. Expression levels of the apoptotic marker active caspase 3, presynaptic protein
SNAP
25 and post‐synaptic protein
PSD
95 were not significantly different between
P
oss
AD
and
ND
groups. There were no significant differences between
ND
and
P
oss
AD
in all of the markers. The horizontal lines in the dot plots represent the values of mean and standard errors. Representative immunoblot images for the proteins
TREM
2,
DAP
12,
IBA
1,
PSD
95,
SNAP
25 and cleaved caspase 3 were compiled in G. The disease groups for the samples in each lane were shown above gel images:
AD
for
A
lzheimer's disease;
PA
for possible
A
lzheimer's disease,
ND
for nondemented normal controls. The blank lane was the lane that molecular weight ladders were loaded. The molecular weights corresponding to each protein were indicated at the right side of the gel images. The brain
TREM
2 has molecular weights of 35–40 kDa.
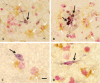
Figure 4
Immunohistochemical characterization of
TREM
2 (triggering receptor expressed by myeloid cells 2) expressions in postmortem human brain tissues. Representative images of double immunohistochemistry of
TREM
2 (dark blue) and
MHCII
(major histocompatibility complex II) (brown) are shown in A–D;
TREM
2 immunoreactive microglia in association with neurons in E–F;
TREM
2 (dark blue) and p‐tau (brown) in G–I; and
TREM
2 and Aβ in J–N. A–D.
TREM
2‐immunoreactive microglia are present in
MHCII
‐negative (arrows) cells and also in
MHCII
‐positive cells (arrow heads). E–I.
TREM
2 immunoreactive microglia are in close contact with neuronal cell bodies (neutral red stained; E, F) in the tissues with minimal
A
lzheimer's disease (
AD
) pathology. Increased
TREM
2 immunoreactivity (dark blue, arrows) could be seen in the areas enriched with p‐tau immunoreactive neurites (brown; G–I). J–N.
TREM
2 immunoreactive microglia (dark blue, arrows; J–N) are observed in the vicinity of amyloid plaques (J–K) and inside amyloid plaques with different compactness of the amyloid aggregates (L–N). All of the micrograph images were taken with 40× objective. The 10‐μm magnification calibration bars were shown in the first picture in each row (A, E, G, J). The brain tissues for the morphological study were
ND
cases in A, B, E; PossAD cases in C, D, F, J, K;
AD
cases in G–I and L–N.
Figure 5
Double immunohistochemistry of
TREM
2 (triggering receptor expressed by myeloid cells 2) with astrocyte and oligodendrocyte markers. Double immunohistochemistry of
TREM
2 with astrocyte marker [glial acidic filament protein (
GFAP
)] or oligodendrocyte‐specific protein shown in this figure were performed in the brain tissues of nondemented controls (
ND
) (A, C), possible
AD
(
P
oss
AD
) (D) and
A
lzheimer's disease (
AD
) (B) cases.
TREM
2 was demonstrated with nickel enhanced
DAB
(DNAX‐activation protein) substrate (dark blue, indicated by black arrows), whereas
GFAP
and oligodendrocyte‐specific protein were demonstrated with
DAB
without enhancement (light brown, indicated by white arrows).
TREM
2 immunoreactivities were not observed in the same cells marked by
GFAP
or oligodendrocytes‐specific protein. Images were taken at 40× objective in A, B, and at 100× with oil immersion lens in C, D. The calibration bars represent 10‐μm length.
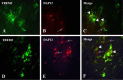
Figure 6
Double immunofluorescence labeling of
TREM
2 (triggering receptor expressed by myeloid cells 2) and
DAP
12 (DNAX‐activation protein 12) in human brain tissues. To illustrate the relationship of
TREM
2 and
DAP
12 expressions in brain cells, results of double immunofluorescence labeling are shown here: A, D:
TREM
2 expression in green; B, E:
DAP
12 expression in red; C, F: overlay of
TREM
2 and
DAP
12 images. In both overlay images, the co‐localization of
TREM
2 and
DAP
12 immunoreactivities were shown (arrow heads). However, some of the
DAP
12 immunoreactive profiles are not associated with
TREM
2 immunoreactivity (indicated by arrows). Magnification: calibration bars: 10 μm.
Similar articles
- What happens to microglial TREM2 in Alzheimer's disease: Immunoregulatory turned into immunopathogenic?
Lue LF, Schmitz C, Walker DG. Lue LF, et al. Neuroscience. 2015 Aug 27;302:138-50. doi: 10.1016/j.neuroscience.2014.09.050. Epub 2014 Oct 2. Neuroscience. 2015. PMID: 25281879 Review. - Investigation of pathology, expression and proteomic profiles in human TREM2 variant postmortem brains with and without Alzheimer's disease.
Toomey CE, Heywood W, Benson BC, Packham G, Mills K, Lashley T. Toomey CE, et al. Brain Pathol. 2020 Jul;30(4):794-810. doi: 10.1111/bpa.12842. Epub 2020 Apr 29. Brain Pathol. 2020. PMID: 32267026 Free PMC article. - Integrated biology approach reveals molecular and pathological interactions among Alzheimer's Aβ42, Tau, TREM2, and TYROBP in Drosophila models.
Sekiya M, Wang M, Fujisaki N, Sakakibara Y, Quan X, Ehrlich ME, De Jager PL, Bennett DA, Schadt EE, Gandy S, Ando K, Zhang B, Iijima KM. Sekiya M, et al. Genome Med. 2018 Mar 29;10(1):26. doi: 10.1186/s13073-018-0530-9. Genome Med. 2018. PMID: 29598827 Free PMC article. - Microglia and Aging: The Role of the TREM2-DAP12 and CX3CL1-CX3CR1 Axes.
Mecca C, Giambanco I, Donato R, Arcuri C. Mecca C, et al. Int J Mol Sci. 2018 Jan 22;19(1):318. doi: 10.3390/ijms19010318. Int J Mol Sci. 2018. PMID: 29361745 Free PMC article. Review. - Distribution and signaling of TREM2/DAP12, the receptor system mutated in human polycystic lipomembraneous osteodysplasia with sclerosing leukoencephalopathy dementia.
Sessa G, Podini P, Mariani M, Meroni A, Spreafico R, Sinigaglia F, Colonna M, Panina P, Meldolesi J. Sessa G, et al. Eur J Neurosci. 2004 Nov;20(10):2617-28. doi: 10.1111/j.1460-9568.2004.03729.x. Eur J Neurosci. 2004. PMID: 15548205
Cited by
- New insights into the role of TREM2 in Alzheimer's disease.
Gratuze M, Leyns CEG, Holtzman DM. Gratuze M, et al. Mol Neurodegener. 2018 Dec 20;13(1):66. doi: 10.1186/s13024-018-0298-9. Mol Neurodegener. 2018. PMID: 30572908 Free PMC article. Review. - TREM2 in the pathogenesis of AD: a lipid metabolism regulator and potential metabolic therapeutic target.
Li RY, Qin Q, Yang HC, Wang YY, Mi YX, Yin YS, Wang M, Yu CJ, Tang Y. Li RY, et al. Mol Neurodegener. 2022 Jun 3;17(1):40. doi: 10.1186/s13024-022-00542-y. Mol Neurodegener. 2022. PMID: 35658903 Free PMC article. Review. - The Transcriptional Regulatory Properties of Amyloid Beta 1-42 may Include Regulation of Genes Related to Neurodegeneration.
Gezen-Ak D, Atasoy İL, Candaş E, Alaylıoğlu M, Dursun E. Gezen-Ak D, et al. Neuromolecular Med. 2018 Sep;20(3):363-375. doi: 10.1007/s12017-018-8498-6. Epub 2018 Jun 12. Neuromolecular Med. 2018. PMID: 29948923 - Imbalance of Microglial TLR4/TREM2 in LPS-Treated APP/PS1 Transgenic Mice: A Potential Link Between Alzheimer's Disease and Systemic Inflammation.
Zhou J, Yu W, Zhang M, Tian X, Li Y, Lü Y. Zhou J, et al. Neurochem Res. 2019 May;44(5):1138-1151. doi: 10.1007/s11064-019-02748-x. Epub 2019 Feb 12. Neurochem Res. 2019. PMID: 30756214
References
- Bianchin MM, Capella HM, Chaves DL, Steindel M, Grisard EC, Ganev GG et al (2004) Nasu‐Hakola disease (polycystic lipomembranous osteodysplasia with sclerosing leukoencephalopathy—PLOSL): a dementia associated with bone cystic lesions. From clinical to genetic and molecular aspects. Cell Mol Neurobiol 24:1–24. - PMC - PubMed
- Borroni B, Ferrari F, Galimberti D, Nacmias B, Barone C, Bagnoli S et al (2013) Heterozygous TREM2 mutations in frontotemporal dementia. Neurobiol Aging 35(4):p934.e7–934.e10. - PubMed
- Bouchon A, Dietrich J, Colonna M (2000) Cutting edge: inflammatory responses can be triggered by TREM‐1, a novel receptor expressed on neutrophils and monocytes. J Immunol 164:4991–4995. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous