New roles for Fc receptors in neurodegeneration-the impact on Immunotherapy for Alzheimer's Disease - PubMed (original) (raw)
Review
New roles for Fc receptors in neurodegeneration-the impact on Immunotherapy for Alzheimer's Disease
James P Fuller et al. Front Neurosci. 2014.
Abstract
There are an estimated 18 million Alzheimer's disease (AD) sufferers worldwide and with no disease modifying treatment currently available, development of new therapies represents an enormous unmet clinical need. AD is characterized by episodic memory loss followed by severe cognitive decline and is associated with many neuropathological changes. AD is characterized by deposits of amyloid beta (Aβ), neurofibrillary tangles, and neuroinflammation. Active immunization or passive immunization against Aβ leads to the clearance of deposits in transgenic mice expressing human Aβ. This clearance is associated with reversal of associated cognitive deficits, but these results have not translated to humans, with both active and passive immunotherapy failing to improve memory loss. One explanation for these observations is that certain anti-Aβ antibodies mediate damage to the cerebral vasculature limiting the top dose and potentially reducing efficacy. Fc gamma receptors (FcγR) are a family of immunoglobulin-like receptors which bind to the Fc portion of IgG, and mediate the response of effector cells to immune complexes. Data from both mouse and human studies suggest that cross-linking FcγR by therapeutic antibodies and the subsequent pro-inflammatory response mediates the vascular side effects seen following immunotherapy. Increasing evidence is emerging that FcγR expression on CNS resident cells, including microglia and neurons, is increased during aging and functionally involved in the pathogenesis of age-related neurodegenerative diseases. Therefore, we propose that increased expression and ligation of FcγR in the CNS, either by endogenous IgG or therapeutic antibodies, has the potential to induce vascular damage and exacerbate neurodegeneration. To produce safe and effective immunotherapies for AD and other neurodegenerative diseases it will be vital to understand the role of FcγR in the healthy and diseased brain. Here we review the literature on FcγR expression, function and proposed roles in multiple age-related neurological diseases. Lessons can be learnt from therapeutic antibodies used for the treatment of cancer where antibodies have been engineered for optimal efficacy.
Keywords: ARIAs; Alzheimer's Disease; Fc receptors; auto-antibodies; cytokines; immunotherapy; neuroinflammation.
Figures
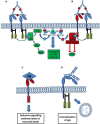
Figure 1
Activation or inhibition of a cell by Fc receptor ligation of IgG immune complexes. (A) Cross linking of activating FcγRs by IgG immune complexes results in the phosphorylation of cytoplasmic ITAM motifs. This allows the recruitment of SH2 domain containing kinases of the SYK family. These kinases activate pathways such as the RAS and PI3K pathways resulting in increased cellular calcium and activation of the cell. (B) The cross linking of an inhibitory receptor to an activating receptor results in the phosphorylation of an ITIM, leading to the recruitment of the phosphatase SHIP1. SHIP1 removes the 5'phopshate from PiP345 inhibiting downstream PI3K signaling, and also interacts with other adaptor proteins to inhibit other pathways. (C) Aβ binds with high affinity to the inhibitory FcγRIIb (_K_D = 5.67 × 10−8 M). Through an unknown signaling pathway, the ligation of Aβ causes the loss of FcγRIIb expressing neurons. (D) FcγRI possesses an extra immunoglobulin like domain compared to other FcγRs. This allows the high affinity binding of monomeric IgG, ligation of monomeric IgG by FcγRI expressing neurons, facilitating antibody uptake.
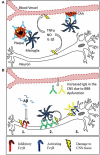
Figure 2
Mechanisms of Fc receptor mediated tissue damage in the CNS. (A) The proposed mechanism for inflammatory tissue damage to CNS vasculature and neurons after Anti-Aβ immunotherapy. Therapeutic antibodies penetrate the CNS and bind to deposits of Aβ in the parenchyma and around the blood vessels. Microglia express activating FcγR, and the antibody-Aβ immune complexes cause cross linking and FcγR activation. This results in a localized inflammatory response causing the vascular side effects observed in mice and humans. Furthermore soluble inflammatory mediators produced by this reaction may interfere with neuronal function or induce damage. (B) There is an emerging role for Fcγ receptors in neurodegeneration, with 3 different proposed mechanisms of Fc receptor mediated damage to neurons. (1) Inhibitory Fcγ receptor (FcγRIIb) expression has been detected on neurons, and FcRIIb binds Aβ with high affinity. The ligation of Aβ by neuronal FcRIIb results in neuronal death. (2) Autoantibodies against neurons are present in the sera of AD patients and also observed binding to neurons. This could lead to FcγR dependant neuronal loss through antibody dependent cellular cytotoxicity, caused by the ligation of activating Fcγ receptors on microglia. (3) Activating FcγR expression has been detected in certain models with neurodegenerative disease. Ligation of IgG by neuronal Fc receptors in mice results in neuronal loss.
Similar articles
- Antibody Engineering for Optimized Immunotherapy in Alzheimer's Disease.
Sumner IL, Edwards RA, Asuni AA, Teeling JL. Sumner IL, et al. Front Neurosci. 2018 Apr 23;12:254. doi: 10.3389/fnins.2018.00254. eCollection 2018. Front Neurosci. 2018. PMID: 29740272 Free PMC article. Review. - Effector function of anti-pyroglutamate-3 Aβ antibodies affects cognitive benefit, glial activation and amyloid clearance in Alzheimer's-like mice.
Crehan H, Liu B, Kleinschmidt M, Rahfeld JU, Le KX, Caldarone BJ, Frost JL, Hettmann T, Hutter-Paier B, O'Nuallain B, Park MA, DiCarli MF, Lues I, Schilling S, Lemere CA. Crehan H, et al. Alzheimers Res Ther. 2020 Jan 13;12(1):12. doi: 10.1186/s13195-019-0579-8. Alzheimers Res Ther. 2020. PMID: 31931873 Free PMC article. - Immunotherapy for Alzheimer's disease: from anti-β-amyloid to tau-based immunization strategies.
Panza F, Frisardi V, Solfrizzi V, Imbimbo BP, Logroscino G, Santamato A, Greco A, Seripa D, Pilotto A. Panza F, et al. Immunotherapy. 2012 Feb;4(2):213-38. doi: 10.2217/imt.11.170. Immunotherapy. 2012. PMID: 22339463 Review. - Gene delivery of a modified antibody to Aβ reduces progression of murine Alzheimer's disease.
Elmer BM, Swanson KA, Bangari DS, Piepenhagen PA, Roberts E, Taksir T, Guo L, Obinu MC, Barneoud P, Ryan S, Zhang B, Pradier L, Yang ZY, Nabel GJ. Elmer BM, et al. PLoS One. 2019 Dec 30;14(12):e0226245. doi: 10.1371/journal.pone.0226245. eCollection 2019. PLoS One. 2019. PMID: 31887144 Free PMC article. - Therapeutic potentials of plant iridoids in Alzheimer's and Parkinson's diseases: A review.
Dinda B, Dinda M, Kulsi G, Chakraborty A, Dinda S. Dinda B, et al. Eur J Med Chem. 2019 May 1;169:185-199. doi: 10.1016/j.ejmech.2019.03.009. Epub 2019 Mar 8. Eur J Med Chem. 2019. PMID: 30877973 Review.
Cited by
- AAV-mediated neuronal expression of an scFv antibody selective for Aβ oligomers protects synapses and rescues memory in Alzheimer models.
Selles MC, Fortuna JTS, Cercato MC, Santos LE, Domett L, Bitencourt ALB, Carraro MF, Souza AS, Janickova H, Azevedo CV, Campos HC, de Souza JM, Alves-Leon S, Prado VF, Prado MAM, Epstein AL, Salvetti A, Longo BM, Arancio O, Klein WL, Sebollela A, De Felice FG, Jerusalinsky DA, Ferreira ST. Selles MC, et al. Mol Ther. 2023 Feb 1;31(2):409-419. doi: 10.1016/j.ymthe.2022.11.002. Epub 2022 Nov 11. Mol Ther. 2023. PMID: 36369741 Free PMC article. - Quantitative systems pharmacology model of the amyloid pathway in Alzheimer's disease: Insights into the therapeutic mechanisms of clinical candidates.
Ramakrishnan V, Friedrich C, Witt C, Sheehan R, Pryor M, Atwal JK, Wildsmith K, Kudrycki K, Lee SH, Mazer N, Hofmann C, Fuji RN, Jin JY, Ramanujan S, Dolton M, Quartino A. Ramakrishnan V, et al. CPT Pharmacometrics Syst Pharmacol. 2023 Jan;12(1):62-73. doi: 10.1002/psp4.12876. Epub 2022 Nov 6. CPT Pharmacometrics Syst Pharmacol. 2023. PMID: 36281062 Free PMC article. - Immune system responses in Parkinson's disease: Early and dynamic.
Tansey MG, Romero-Ramos M. Tansey MG, et al. Eur J Neurosci. 2019 Feb;49(3):364-383. doi: 10.1111/ejn.14290. Epub 2018 Dec 10. Eur J Neurosci. 2019. PMID: 30474172 Free PMC article. Review. - AAV Vector-Mediated Antibody Delivery (A-MAD) in the Central Nervous System.
Marino M, Holt MG. Marino M, et al. Front Neurol. 2022 Apr 12;13:870799. doi: 10.3389/fneur.2022.870799. eCollection 2022. Front Neurol. 2022. PMID: 35493843 Free PMC article. Review. - Fc gamma receptors are expressed in the developing rat brain and activate downstream signaling molecules upon cross-linking with immune complex.
Stamou M, Grodzki AC, van Oostrum M, Wollscheid B, Lein PJ. Stamou M, et al. J Neuroinflammation. 2018 Jan 6;15(1):7. doi: 10.1186/s12974-017-1050-z. J Neuroinflammation. 2018. PMID: 29306331 Free PMC article.
References
- Adolfsson O., Pihlgren M., Toni N., Varisco Y., Buccarello A. L., Antoniello K., et al. (2012). An effector-reduced anti-β-amyloid (Aβ) antibody with unique aβ binding properties promotes neuroprotection and glial engulfment of Aβ. J. Neurosci. 32, 9677–9689 10.1523/JNEUROSCI.4742-11.2012 - DOI - PMC - PubMed
- Allsop D., Wong C. W., Ikeda S., Landon M., Kidd M., Glenner G. G. (1988). Evidence for the origin of cerebral amyloid in Alzheimer's disease drom A Beta protein precursor. Neuropathol. Appl. Neurobiol. 14, 254–255
- Andoh T., Kuraishi Y. (2004). Primary sensory neurons express the high affinity IgG Fc gamma RI receptor and responds to IgG-antigen complex. J. Pharmacol. Sci. 94, 74P 10.1096/fj.02-1169fje - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources