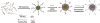Nanocarrier mediated delivery of siRNA/miRNA in combination with chemotherapeutic agents for cancer therapy: current progress and advances - PubMed (original) (raw)
Review
Nanocarrier mediated delivery of siRNA/miRNA in combination with chemotherapeutic agents for cancer therapy: current progress and advances
Nishant S Gandhi et al. J Control Release. 2014.
Abstract
Chemotherapeutic agents have certain limitations when it comes to treating cancer, the most important being severe side effects along with multidrug resistance developed against them. Tumor cells exhibit drug resistance due to activation of various cellular level processes viz. activation of drug efflux pumps, anti-apoptotic defense mechanisms, etc. Currently, RNA interference (RNAi) based therapeutic approaches are under vibrant scrutinization to seek cancer cure. Especially small interfering RNA (siRNA) and micro RNA (miRNA), are able to knock down the carcinogenic genes by targeting the mRNA expression, which underlies the uniqueness of this therapeutic approach. Recent research focus in the regime of cancer therapy involves the engagement of targeted delivery of siRNA/miRNA in combinations with other therapeutic agents (such as gene, DNA or chemotherapeutic drug) for targeting permeability glycoprotein (P-gp), multidrug resistant protein 1 (MRP-1), B-cell lymphoma (BCL-2) and other targets that are mainly responsible for resistance in cancer therapy. RNAi-chemotherapeutic drug combinations have also been found to be effective against different molecular targets as well and can increase the sensitization of cancer cells to therapy several folds. However, due to stability issues associated with siRNA/miRNA suitable protective carrier is needed and nanotechnology based approaches have been widely explored to overcome these drawbacks. Furthermore, it has been univocally advocated that the co-delivery of siRNA/miRNA with other chemodrugs significantly enhances their capability to overcome cancer resistance compared to naked counterparts. The objective of this article is to review recent nanocarrier based approaches adopted for the delivery of siRNA/miRNA combinations with other anticancer agents (siRNA/miRNA/pDNA/chemodrugs) to treat cancer.
Keywords: Cancer; Clinical trial; Combination therapy; Nanotechnology; miRNA; siRNA.
Copyright © 2014 Elsevier B.V. All rights reserved.
Figures
Figure 1
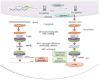
RNA interference mechanism: siRNA – The siRNA pathway begins with cleavage of dsRNA by enzyme DICER resulting in siRNA in the cytoplasm of cell [34, 49]. The siRNA then binds to Argonaute (AGO2) protein and RNA inducing silencing complex (RISC)[37]. One strand of the siRNA duplex (the passenger strand) is removed by AGO2 resulting in RISC containing guide strand [50]. The activated RISC-siRNA binds to the complementary sequences on the mRNA and results in its cleavage and degradation [51]. Biogenesis of miRNA – The RNA polymerase II or III are responsible for the production of primary-miRNA’s (pri-miRNA) [36, 52]. In the nucleus, the resulting pri-miRNA’s are cleaved by the microprocessor complex Drosha [53]. The pre-miRNA is transported to the cytoplasm by Exportin 5(XPO5) and the loop structure is removed by the Dicer complex (Dicer – TAR binding protein) resulting in miRNA or miRNA duplexes [54, 55]. One strand of the duplex is incorporated into AGO2 and RISC which targets mRNA and results in its degradation[56]. (Adapted with permission from ref.[57]).
Figure 2
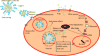
Mechanism of sensitization of resistant cancer cells by co-delivering siRNA and a chemotherapeutic agent. Therapeutic agents encapsulated in nanoparticles evade the efflux pump via endosomal internalization. Once in the endosome, the specifically designed nanoparticles releases siRNA/miRNA and drug in the cytosol resulting in the cytotoxic effect.
Figure 3
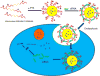
Schematic representation of non-targeted and targeted LCP nanoparticles adapted with permission from ref. [48].
Figure 4. Schematic illustration of the formation of mixed micellar system using G (4)-PAMAM-D-PEG-DOPE/PEG-DOPE mixed micellar system
A poly (ethylene glycol) – dioleoylphosphatidyl ethanolamine (PEG-DOPE) modified G (4)-PAMAM nanocarrier used to deliver siRNA targeting green fluorescence protein. (Adapted with permission from ref. [138])
Figure 5
Schematic representation of self-assembled cationic micelles of PDMAEMA–PCL– PDMAEMA triblock copolymers for the simultaneous combinatorial delivery of PTX and siRNA. The figure depicts the release of siRNA from the cationic micelles inside the cell and degradation of mRNA resulting in its action.
Figure 6
Schematic representation of cationic solid lipid nanoparticles complexed with siRNA A) Empty solid lipid nanoparticles B) PTX loaded solid lipid nanoparticles (adapted with permission from ref. [147])
Figure 7
Schematic illustration of the formation of multifunctional nanoassemblies comprising of DOX and siRNA. (Adapted with permission from ref.[208].
Figure 8
A Schematic illustration of the formation of micellar nanoparticles. (Adapted with permission from ref. [148])
Figure 9
Lipid Nanoparticle for systemic delivery (Adapted from online alnylam pharmaceuticals)
Similar articles
- Combinatorial therapeutic approaches with RNAi and anticancer drugs using nanodrug delivery systems.
Babu A, Munshi A, Ramesh R. Babu A, et al. Drug Dev Ind Pharm. 2017 Sep;43(9):1391-1401. doi: 10.1080/03639045.2017.1313861. Epub 2017 May 19. Drug Dev Ind Pharm. 2017. PMID: 28523942 Free PMC article. - Simultaneous nanocarrier-mediated delivery of siRNAs and chemotherapeutic agents in cancer therapy and diagnosis: Recent advances.
Bidar N, Darroudi M, Ebrahimzadeh A, Safdari M, de la Guardia M, Baradaran B, Goodarzi V, Oroojalian F, Mokhtarzadeh A. Bidar N, et al. Eur J Pharmacol. 2022 Jan 15;915:174639. doi: 10.1016/j.ejphar.2021.174639. Epub 2021 Dec 14. Eur J Pharmacol. 2022. PMID: 34919890 Review. - RNAi-combined nano-chemotherapeutics to tackle resistant tumors.
Tekade RK, Tekade M, Kesharwani P, D'Emanuele A. Tekade RK, et al. Drug Discov Today. 2016 Nov;21(11):1761-1774. doi: 10.1016/j.drudis.2016.06.029. Epub 2016 Jul 2. Drug Discov Today. 2016. PMID: 27380716 Review. - Chemotherapy with si-RNA and Anti-Cancer Drugs.
Kaur K, Rath G, Chandra S, Singh R, Goyal AK. Kaur K, et al. Curr Drug Deliv. 2018;15(3):300-311. doi: 10.2174/1567201814666170518141440. Curr Drug Deliv. 2018. PMID: 28521675 Review. - The importance of co-delivery of nanoparticle-siRNA and anticancer agents in cancer therapy.
Khelghati N, Soleimanpour Mokhtarvand J, Mir M, Alemi F, Asemi Z, Sadeghpour A, Maleki M, Samadi Kafil H, Jadidi-Niaragh F, Majidinia M, Yousefi B. Khelghati N, et al. Chem Biol Drug Des. 2021 Apr;97(4):997-1015. doi: 10.1111/cbdd.13824. Epub 2021 Feb 18. Chem Biol Drug Des. 2021. PMID: 33458952 Review.
Cited by
- Engineered Mesenchymal Stem Cells as an Anti-Cancer Trojan Horse.
Nowakowski A, Drela K, Rozycka J, Janowski M, Lukomska B. Nowakowski A, et al. Stem Cells Dev. 2016 Oct;25(20):1513-1531. doi: 10.1089/scd.2016.0120. Epub 2016 Sep 7. Stem Cells Dev. 2016. PMID: 27460260 Free PMC article. - DNA Polyplexes as Combinatory Drug Carriers of Doxorubicin and Cisplatin: An in Vitro Study.
Kang HC, Cho H, Bae YH. Kang HC, et al. Mol Pharm. 2015 Aug 3;12(8):2845-57. doi: 10.1021/mp500873k. Epub 2015 Jul 13. Mol Pharm. 2015. PMID: 26132975 Free PMC article. - P-glycoprotein-targeted photodynamic therapy boosts cancer nanomedicine by priming tumor microenvironment.
Mao C, Li F, Zhao Y, Debinski W, Ming X. Mao C, et al. Theranostics. 2018 Nov 29;8(22):6274-6290. doi: 10.7150/thno.29580. eCollection 2018. Theranostics. 2018. PMID: 30613297 Free PMC article. - miR-622 is a novel potential biomarker of breast carcinoma and impairs motility of breast cancer cells through targeting NUAK1 kinase.
Orlandella FM, Mariniello RM, Mirabelli P, De Stefano AE, Iervolino PLC, Lasorsa VA, Capasso M, Giannatiempo R, Rongo M, Incoronato M, Messina F, Salvatore M, Soricelli A, Salvatore G. Orlandella FM, et al. Br J Cancer. 2020 Aug;123(3):426-437. doi: 10.1038/s41416-020-0884-9. Epub 2020 May 18. Br J Cancer. 2020. PMID: 32418991 Free PMC article. - MicroRNA-495 suppresses cell proliferation and invasion of hepatocellular carcinoma by directly targeting insulin-like growth factor receptor-1.
Ye Y, Zhuang J, Wang G, He S, Zhang S, Wang G, Ni J, Wang J, Xia W. Ye Y, et al. Exp Ther Med. 2018 Jan;15(1):1150-1158. doi: 10.3892/etm.2017.5467. Epub 2017 Nov 8. Exp Ther Med. 2018. PMID: 29434703 Free PMC article. Retracted.
References
- Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, Rosso S, Coebergh JW, Comber H, Forman D, Bray F. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. European journal of cancer (Oxford, England: 1990) 2013;49:1374–1403. - PubMed
- Ortiz-Tudela E, Mteyrek A, Ballesta A, Innominato PF, Levi F. Cancer chronotherapeutics: experimental, theoretical, and clinical aspects. Handbook of experimental pharmacology. 2013:261–288. - PubMed
- Tekade RK, Dutta T, Tyagi A, Bharti AC, Das BC, Jain NK. Surface-engineered dendrimers for dual drug delivery: a receptor up-regulation and enhanced cancer targeting strategy. Journal of drug targeting. 2008;16:758–772. - PubMed
- Malmstrom PU, Rintala E, Wahlqvist R, Hellstrom P, Hellsten S, Hannisdal E. Five-year Followup of a Prospective Trial of Radical Cystectomy and Neoadjuvant Chemotherapy: Nordic Cystectomy Trial 1. The Journal of Urology. 1996;155:1903–1906. - PubMed
- Steinberg JL, Yeo W, Zhong S, Chan JYH, Tam JS, Chan PKS, Leung NWY, Johnson PJ. Hepatitis B virus reactivation in patients undergoing cytotoxic chemotherapy for solid tumours: Precore/core mutations may play an important role. Journal of medical virology. 2000;60:249–255. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous