Oxidative stress induces autophagy in response to multiple noxious stimuli in retinal ganglion cells - PubMed (original) (raw)
Review
Oxidative stress induces autophagy in response to multiple noxious stimuli in retinal ganglion cells
Wen-jian Lin et al. Autophagy. 2014.
Abstract
Retinal ganglion cells (RGCs) are the only afferent neurons that can transmit visual information to the brain. The death of RGCs occurs in the early stages of glaucoma, diabetic retinopathy, and many other retinal diseases. Autophagy is a highly conserved lysosomal pathway, which is crucial for maintaining cellular homeostasis and cell survival under stressful conditions. Research has established that autophagy exists in RGCs after increasing intraocular pressure (IOP), retinal ischemia, optic nerve transection (ONT), axotomy, or optic nerve crush. However, the mechanism responsible for defining how autophagy is induced in RGCs has not been elucidated. Accumulating data has pointed to an essential role of reactive oxygen species (ROS) in the activation of autophagy. RGCs have long axons with comparatively high densities of mitochondria. This makes them more sensitive to energy deficiency and vulnerable to oxidative stress. In this review, we explore the role of oxidative stress in the activation of autophagy in RGCs, and discuss the possible mechanisms that are involved in this process. We aim to provide a more theoretical basis of oxidative stress-induced autophagy, and provide innovative targets for therapeutic intervention in retinopathy.
Figures
Figure 1. Early and late stage autophagy in retinal ganglion cell layers. In the earlier stage, the double-membrane-bound structure (see arrow) is fusing with a lysosome (shown by the arrowhead). At the later stages (see asterisk), fusion with the lysosome has occurred, and the structure (an autolysosome) is undergoing proteolysis. Scale bar: 500 nm.
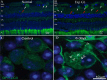
Figure 2. Autophagy is activated in RGCs. (A and B) Comparison of normal retinas (A) with experimentally-induced glaucomatous retinas (Exp GL, B). LC3 immunoreactivity is observed as clusters of small, intensely stained green granules. A markedly increased number of positive granules are visible in the cytosol of the RGC in the GCL (white arrows) and in the dendrites of the RGC in the IPL (red arrows). (C and D) RGCs in control retina and at 6 d after ONT. Cells from control retinas display a diffuse and homogeneous GFP-LC3 green fluorescence in the cytosol, whereas the axotomized RGCs in the right panel show visible GFP-LC3 dots in the cytosol that are identified as autophagosomes, and indicated by arrowheads. (A and B) Scale bar: 20 μm; (C and D) scale bar: 10 μm.
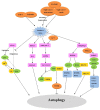
Figure 3. Regulation of autophagy by oxidative stress in RGCs. Various injuries cause oxidative stress in RGCs by primary and secondary degeneration, and thereby induce autophagy. ATG4 has 2 effects: A) it primes ATG8 homologs for conjugation with PE, allowing for lipidated LC3 and GABARAP family protein incorporation into the phagophore membrane, and B) deconjugation of LC3 and GABARAP protein from phosphatidylethanolamine apparently permits recycling of these proteins. Increased concentrations of ROS inactivate the second function of ATG4. In response to oxidative stress, HIF-1 is activated, which indirectly augments NMNAT via induction of IL6. Additionally, NMNAT may act on the upstream pathways of autophagy. Oxidative stress and increasing intracellular Ca2+ levels will activate AMPK, which promotes the formation of a TSC1-TSC2 complex, leading to MTOR inactivation and initiation of autophagy. Under oxidative stress, mitophagy is activated. BNIP3L localizes to the mitochondrial outer membrane, it interacts directly with LC3 or GABARAP, and mediates the recruitment of damaged mitochondria to phagophores. BNIP3L also competes with BECN1 for binding to BCL2 and thereby releases BECN1 to induce autophagy. PINK1 recruits the E3 ligase PARK2 to damaged mitochondria, which then mediates mitochondrial fragmentation and targeting to phagophores, which is followed by ubiquitination of VDAC1 and binding to SQSTM1. Then SQSTM1 targets this complex to the autophagosome by interacting with the autophagic protein LC3.
Similar articles
- Mitochondria in Retinal Ganglion Cells: Unraveling the Metabolic Nexus and Oxidative Stress.
Yang TH, Kang EY, Lin PH, Yu BB, Wang JH, Chen V, Wang NK. Yang TH, et al. Int J Mol Sci. 2024 Aug 7;25(16):8626. doi: 10.3390/ijms25168626. Int J Mol Sci. 2024. PMID: 39201313 Free PMC article. Review. - Autophagy promotes survival of retinal ganglion cells after optic nerve axotomy in mice.
Rodríguez-Muela N, Germain F, Mariño G, Fitze PS, Boya P. Rodríguez-Muela N, et al. Cell Death Differ. 2012 Jan;19(1):162-9. doi: 10.1038/cdd.2011.88. Epub 2011 Jun 24. Cell Death Differ. 2012. PMID: 21701497 Free PMC article. - Autophagy dysregulation and the fate of retinal ganglion cells in glaucomatous optic neuropathy.
Russo R, Nucci C, Corasaniti MT, Bagetta G, Morrone LA. Russo R, et al. Prog Brain Res. 2015;220:87-105. doi: 10.1016/bs.pbr.2015.04.009. Epub 2015 Jul 2. Prog Brain Res. 2015. PMID: 26497786 Review.
Cited by
- The Relevance of Oxidative Stress in the Pathogenesis and Therapy of Retinal Dystrophies.
B Domènech E, Marfany G. B Domènech E, et al. Antioxidants (Basel). 2020 Apr 23;9(4):347. doi: 10.3390/antiox9040347. Antioxidants (Basel). 2020. PMID: 32340220 Free PMC article. Review. - Mitochondrion-associated protein peroxiredoxin 3 promotes benign prostatic hyperplasia through autophagy suppression and pyroptosis activation.
Jiang MY, Han ZD, Li W, Yue F, Ye J, Li B, Cai Z, Lu JM, Dong W, Jiang X, Zhong W, He H, Liu L. Jiang MY, et al. Oncotarget. 2017 May 17;8(46):80295-80302. doi: 10.18632/oncotarget.17927. eCollection 2017 Oct 6. Oncotarget. 2017. PMID: 29113303 Free PMC article. - Immunosubunit β5i Knockout Suppresses Neovascularization and Restores Autophagy in Retinal Neovascularization by Targeting ATG5 for Degradation.
Ji L, Li L, Zhao Y, Liu S, Li J, Zhang J, Zhao Q, Wang S. Ji L, et al. Invest Ophthalmol Vis Sci. 2020 Dec 1;61(14):30. doi: 10.1167/iovs.61.14.30. Invest Ophthalmol Vis Sci. 2020. PMID: 33369639 Free PMC article. - The Role of Endogenous Neuroprotective Mechanisms in the Prevention of Retinal Ganglion Cells Degeneration.
Pietrucha-Dutczak M, Amadio M, Govoni S, Lewin-Kowalik J, Smedowski A. Pietrucha-Dutczak M, et al. Front Neurosci. 2018 Nov 15;12:834. doi: 10.3389/fnins.2018.00834. eCollection 2018. Front Neurosci. 2018. PMID: 30524222 Free PMC article. Review. - Morphine-induced RACK1-dependent autophagy in immortalized neuronal cell lines.
Liu LT, Song YQ, Chen XS, Liu Y, Zhu JJ, Zhou LM, Xu SJ, Wan LH. Liu LT, et al. Br J Pharmacol. 2020 Apr;177(7):1609-1621. doi: 10.1111/bph.14922. Epub 2020 Jan 30. Br J Pharmacol. 2020. PMID: 31747048 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources