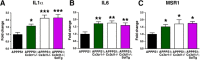Opposing effects of membrane-anchored CX3CL1 on amyloid and tau pathologies via the p38 MAPK pathway - PubMed (original) (raw)
Opposing effects of membrane-anchored CX3CL1 on amyloid and tau pathologies via the p38 MAPK pathway
Sungho Lee et al. J Neurosci. 2014.
Abstract
Several Alzheimer's disease (AD) risk genes are specifically expressed by microglia within the CNS. However, the mechanisms by which microglia regulate the pathological hallmarks of AD--extracellular deposition of β-amyloid (Aβ) and intraneuronal hyperphosphorylation of microtubule-associated protein tau (MAPT)--remain to be established. Notably, deficiency for the microglial CX3CR1 receptor has opposing effects on Aβ and MAPT pathologies. CX3CL1, the neuronally derived cognate ligand for CX3CR1, signals both in membrane-anchored and soluble forms. In this study, we sought to determine the relative contribution on membrane-anchored versus soluble CX3CL1 in regulating the microglia-mediated amelioration of Aβ pathology, as well as provide insight into the potential downstream microglial-based mechanisms. As expected, CX3CL1 deficiency reduced Aβ deposition in APPPS1 animals in a similar manner to CX3CR1 deficiency. Surprisingly, however, CX3CL1-deficient APPPS1 animals exhibited enhanced neuronal MAPT phosphorylation despite reduced amyloid burden. Importantly, neither of these phenotypes was altered by transgenic expression of the soluble CX3CL1 isoform, suggesting that it is the membrane-anchored version of CX3CL1 that regulates microglial phagocytosis of Aβ and neuronal MAPT phosphorylation. Analysis of transcript levels in purified microglia isolated from APPPS1 mice with the various CX3CL1/CX3CR1 genotypes revealed increased expression of inflammatory cytokines and phagocytic markers, which was associated with activation of p38 mitogen-activated protein kinase and Aβ internalization within microglia. Together, these studies challenge the "frustrated phagocytosis" concept and suggest that neuronal-microglial communication link the two central AD pathologies.
Keywords: Alzheimer's disease; CX3CL1; microglia; phagocytosis.
Copyright © 2014 the authors 0270-6474/14/3412538-09$15.00/0.
Figures
Figure 1.
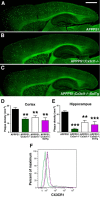
Unaltered fibrillar Aβ deposition in APPPS1 mice expressing obligate soluble CX3CL1. A–C, Brain sections from APPPS1 (n = 6; A), APPPS1;_Cx3cl1_−/− (n = 6; B), and APPPS1;_Cx3cl1_−/−;SolTg (n = 6; C) mice at 4 months of age were stained with thioflavine S. A series of low-power images (Scale bar, 1 mm) were used to reconstruct the cortex and hippocampus from three sections per animal. A, B, D, E, APPPS1;_Cx3cl1_−/− mice (B) exhibited reduced fibrillar Aβ deposition in the cortex (D) and the hippocampus (E) compared with age-matched APPPS1 controls (A). C–E, However, expression of SolTg in APPPS1;_Cx3cl1_−/− mice (C) did not additionally alter fibrillar Aβ deposition in either brain regions (D, E). Quantification of fibrillar Aβ deposition in APPPS1;_Cx3cr1_−/− mice (n = 7) from a previous study (Lee et al., 2010) is included as reference (D, E; **p < 0.01, ***p < 0.001, 1-way ANOVA with Newman–Keuls post hoc test). F, To determine the effect of SolTg on membrane CX3CR1 expression, microglia isolated from APPPS1 (black), APPPS1;_Cx3cr1_−/− (green), and APPPS1;_Cx3cl1_−/−;SolTg mice (purple) were analyzed by flow cytometry. CD11b+ gated cells from APPPS1 and APPPS1;_Cx3cl1_−/−;SolTg brains expressed comparable levels of membrane CX3CR1, whereas no CX3CR1 expression was detected in APPPS1;_Cx3cr1_−/− cells. These are the result of two experiments.
Figure 2.
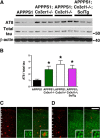
Hyperphosphorylation of MAPT in APPPS1 mice lacking membrane-anchored CX3CL1 signaling. A, Western blots of cortical lysates from APPPS1 (n = 6), APPPS1;_Cx3cr1_−/− (n = 5), APPPS1;_Cx3cl1_−/− (n = 4), and APPPS1;_Cx3cl1_−/−;SolTg mice (n = 4) at 4 months were probed with antibodies against phospho-MAPT (AT8), total MAPT, and β-actin. B, Quantification of band intensities revealed significant increases in AT8-reactive MAPT relative to total MAPT expression in APPPS1;_Cx3cr1_−/−, APPPS1;_Cx3cl1_−/−, and APPPS1;_Cx3cl1_−/−;SolTg genotypes compared with APPPS1 controls. C, D, Immunostaining brain sections for Aβ with monoclonal 4G8 antibody (red) and AT8 antibody (green) revealed pronounced phospho-MAPT accumulation within layer IV/V cortical pyramidal neurons of APPPS1;_Cx3cl1_−/− mice (D, insets), which was not observed in APPPS1 controls (C, insets). Scale bar, 100 μm; *p < 0.05, one-way ANOVA with Newman–Keuls post hoc test.
Figure 3.
Altered expression of inflammatory markers in microglia. A–C, Relative expression of IL1α (A), IL6 (B), and MSR1 mRNA (C) in microglia isolated from 4-month-old APPPS1 (n = 10), APPPS1;_Cx3cr1_−/− (n = 8–10), APPPS1;_Cx3cl1_−/− (n = 8), and APPPS1;_Cx3cl1_−/−;SolTg animals (n = 8). The mRNA levels for each gene were normalized to mRNA levels of GAPDH, and expressed relative to that of APPPS1 mice. *p < 0.05, **p < 0.01, ***p < 0.001, one-way ANOVA with Newman–Keuls post hoc test.
Figure 4.
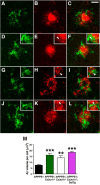
Enhanced microglial Aβ phagocytosis in APPPS1 mice lacking membrane-anchored CX3CL1 signaling. A–L, Brain sections from 4-month-old APPPS1 (A–C) and APPPS1;_Cx3cr1_−/− (D–F), APPPS1;_Cx3cl1_−/− (G–I), and APPPS1;_Cx3cl1_−/−;SolTg mice (J–L) were immunostained with the antibody against the microglial marker Iba1 (A, C, D, F, G, I, J, L, green) and the monoclonal Aβ antibody 4G8 (B, C, E, F, H, I, K, L, red). C, E, F, K, L, M, Quantification of Aβ phagocytosis (M) revealed increased intracellular Aβ volume per cell in APPPS1;_Cx3cr1_−/− (E, F, insets, arrowheads), APPPS1;_Cx3cl1_−/− (H, I, insets, arrowheads), and APPPS1;_Cx3cl1_−/−;SolTg mice (K, L, insets, arrowheads) compared with APPPS1 controls (C). Scale bar, 25 μm; **p < 0.01, ***p < 0.001, one-way ANOVA with Newman–Keuls post hoc test.
Figure 5.
Increased p38 MAPK activation in APPPS1 mice lacking membrane-anchored CX3CL1 signaling. A–C, Immunostaining with the antibody against CD45, a marker for cells of hematopoietic lineage upregulated in activated microglia (A, C, red), the antibody against phospho-p38 MAPK (B, C, green), and the monoclonal Aβ antibody 4G8 (A–C, blue) revealed predominant localization of phospho-p38 MAPK within plaque-adjacent microglia in APPPS1 mice at 4 months (C). D, Western blots of cortical lysates from APPPS1 (n = 4), APPPS1;_Cx3cr1_−/− (n = 5), APPPS1;_Cx3cl1_−/− (n = 4), and APPPS1;_Cx3cl1_−/−;SolTg mice (n = 4) at 4 months were probed with anti-phospho-p38 MAPK and total p38 MAPK antibodies. F, Quantification of band intensities revealed increased phopho-p38 MAPK levels relative to total p38 MAPK levels in APPPS1;_Cx3cr1_−/−, APPPS1;_Cx3cl1_−/−, and APPPS1;_Cx3cl1_−/−;SolTg mice compared with APPPS1 controls. E, G, In addition, Western blot analysis of cortical lysates for phospho-MAPKAPK2 expression relative to β-actin (E) revealed significant increases in APPPS1;_Cx3cr1_−/− (n = 5) and APPPS1;_Cx3cl1_−/−;SolTg mice (n = 4) compared with APPPS1 controls (n = 6; G). Scale bar, 25 μm; *p < 0.05, **p < 0.01, one-way ANOVA with Newman–Keuls post hoc test.
Similar articles
- Effects of CX3CR1 and Fractalkine Chemokines in Amyloid Beta Clearance and p-Tau Accumulation in Alzheimer's Disease (AD) Rodent Models: Is Fractalkine a Systemic Biomarker for AD?
Merino JJ, Muñetón-Gómez V, Alvárez MI, Toledano-Díaz A. Merino JJ, et al. Curr Alzheimer Res. 2016;13(4):403-12. doi: 10.2174/1567205013666151116125714. Curr Alzheimer Res. 2016. PMID: 26567742 Review. - CX3CR1 deficiency alters microglial activation and reduces beta-amyloid deposition in two Alzheimer's disease mouse models.
Lee S, Varvel NH, Konerth ME, Xu G, Cardona AE, Ransohoff RM, Lamb BT. Lee S, et al. Am J Pathol. 2010 Nov;177(5):2549-62. doi: 10.2353/ajpath.2010.100265. Epub 2010 Sep 23. Am J Pathol. 2010. PMID: 20864679 Free PMC article. - CX3CL1 Pathway as a Molecular Target for Treatment Strategies in Alzheimer's Disease.
Bivona G, Iemmolo M, Ghersi G. Bivona G, et al. Int J Mol Sci. 2023 May 4;24(9):8230. doi: 10.3390/ijms24098230. Int J Mol Sci. 2023. PMID: 37175935 Free PMC article. Review. - Heterozygous CX3CR1 Deficiency in Microglia Restores Neuronal β-Amyloid Clearance Pathways and Slows Progression of Alzheimer's Like-Disease in PS1-APP Mice.
Hickman SE, Allison EK, Coleman U, Kingery-Gallagher ND, El Khoury J. Hickman SE, et al. Front Immunol. 2019 Dec 2;10:2780. doi: 10.3389/fimmu.2019.02780. eCollection 2019. Front Immunol. 2019. PMID: 31849963 Free PMC article. - Fibrillar Aβ triggers microglial proteome alterations and dysfunction in Alzheimer mouse models.
Sebastian Monasor L, Müller SA, Colombo AV, Tanrioever G, König J, Roth S, Liesz A, Berghofer A, Piechotta A, Prestel M, Saito T, Saido TC, Herms J, Willem M, Haass C, Lichtenthaler SF, Tahirovic S. Sebastian Monasor L, et al. Elife. 2020 Jun 8;9:e54083. doi: 10.7554/eLife.54083. Elife. 2020. PMID: 32510331 Free PMC article.
Cited by
- Blocking postsynaptic density-93 binding to C-X3-C motif chemokine ligand 1 promotes microglial phenotypic transformation during acute ischemic stroke.
Cao XW, Yang H, Liu XM, Lou SY, Kong LP, Rong LQ, Shan JJ, Xu Y, Zhang QX. Cao XW, et al. Neural Regen Res. 2023 May;18(5):1033-1039. doi: 10.4103/1673-5374.355759. Neural Regen Res. 2023. PMID: 36254989 Free PMC article. - _Cx3cr1-_deficient microglia exhibit a premature aging transcriptome.
Gyoneva S, Hosur R, Gosselin D, Zhang B, Ouyang Z, Cotleur AC, Peterson M, Allaire N, Challa R, Cullen P, Roberts C, Miao K, Reynolds TL, Glass CK, Burkly L, Ransohoff RM. Gyoneva S, et al. Life Sci Alliance. 2019 Dec 2;2(6):e201900453. doi: 10.26508/lsa.201900453. Print 2019 Dec. Life Sci Alliance. 2019. PMID: 31792059 Free PMC article. - Increased inflammation in BA21 brain tissue from African Americans with Alzheimer's disease.
Ferguson SA, Varma V, Sloper D, Panos JJ, Sarkar S. Ferguson SA, et al. Metab Brain Dis. 2020 Jan;35(1):121-133. doi: 10.1007/s11011-019-00512-2. Epub 2019 Dec 10. Metab Brain Dis. 2020. PMID: 31823110 - Early chronic suppression of microglial p38α in a model of Alzheimer's disease does not significantly alter amyloid-associated neuropathology.
Braun DJ, Frazier HN, Davis VA, Coleman MJ, Rogers CB, Van Eldik LJ. Braun DJ, et al. PLoS One. 2023 May 31;18(5):e0286495. doi: 10.1371/journal.pone.0286495. eCollection 2023. PLoS One. 2023. PMID: 37256881 Free PMC article. - Carnosine modulates Aβ-induced transcriptional aberrations in murine microglial cells.
Rivi V, Carota G, Tascedda F, Blom JMC, Caraci F, Benatti C, Caruso G. Rivi V, et al. Curr Res Pharmacol Drug Discov. 2025 May 6;8:100221. doi: 10.1016/j.crphar.2025.100221. eCollection 2025. Curr Res Pharmacol Drug Discov. 2025. PMID: 40487590 Free PMC article.
References
- Chakrabarty P, Jansen-West K, Beccard A, Ceballos-Diaz C, Levites Y, Verbeeck C, Zubair AC, Dickson D, Golde TE, Das P. Massive gliosis induced by interleukin-6 suppresses Abeta deposition in vivo: evidence against inflammation as a driving force for amyloid deposition. FASEB J. 2010a;24:548–559. doi: 10.1096/fj.09-141754. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS074804/NS/NINDS NIH HHS/United States
- AG023012/AG/NIA NIH HHS/United States
- NS067431/NS/NINDS NIH HHS/United States
- NS047804/NS/NINDS NIH HHS/United States
- F30 NS068003/NS/NINDS NIH HHS/United States
- R01 AG023012/AG/NIA NIH HHS/United States
- T32 NS067431/NS/NINDS NIH HHS/United States
- T32 GM007250/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous