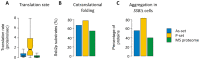Global analysis of protein aggregation in yeast during physiological conditions and arsenite stress - PubMed (original) (raw)
Global analysis of protein aggregation in yeast during physiological conditions and arsenite stress
Sebastian Ibstedt et al. Biol Open. 2014.
Abstract
Protein aggregation is a widespread phenomenon in cells and associated with pathological conditions. Yet, little is known about the rules that govern protein aggregation in living cells. In this study, we biochemically isolated aggregation-prone proteins and used computational analyses to identify characteristics that are linked to physiological and arsenite-induced aggregation in living yeast cells. High protein abundance, extensive physical interactions, and certain structural properties are positively correlated with an increased aggregation propensity. The aggregated proteins have high translation rates and are substrates of ribosome-associated Hsp70 chaperones, indicating that they are susceptible for aggregation primarily during translation/folding. The aggregation-prone proteins are enriched for multiple chaperone interactions, thus high protein abundance is probably counterbalanced by molecular chaperones to allow soluble expression in vivo. Our data support the notion that arsenite interferes with chaperone activity and indicate that arsenite-aggregated proteins might engage in extensive aberrant protein-protein interactions. Expression of aggregation-prone proteins is down-regulated during arsenite stress, possibly to prevent their toxic accumulation. Several aggregation-prone yeast proteins have human homologues that are implicated in misfolding diseases, suggesting that similar mechanisms may apply in disease- and non-disease settings.
Keywords: arsenite; chaperone; protein aggregation; protein folding; translation.
© 2014. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interests: The authors have no competing interests to declare.
Figures
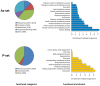
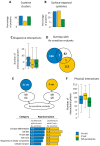
Fig. 1.. Functional characteristics of aggregation-prone proteins.
Biological processes that are significantly enriched in the As- and P-sets compared to the S. cerevisiae genome. Circle diagrams indicate the distribution of aggregated proteins into functional categories where “Translation” excludes ribosomal proteins. Bar diagrams indicate the fold-enrichment of functional categories compared to the genome using GO data from SGD. All shown categories are significant with 5% FDR.
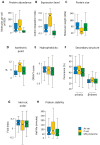
Fig. 2.. Physico-chemical characteristics of aggregation-prone proteins.
(A) Molecules per cell. The abundance of proteins in each set during non-stress conditions is plotted. Proteins in the P-set are significantly more abundant than proteins in the As-set (p = 0.017, U = 3907.5), whilst proteins in the MS proteome are less abundant than those in the As-set (p < 10−15, _U_ = 100021) and P-set (_p_ < 10−15, _U_ = 69411). (B) Expression levels. The codon adaptation index is an indication of gene expression levels (Sharp and Li, 1987), and the CAI for proteins in each data-set are displayed. The CAI for the P-set is significantly higher than for the As-set (_p_ = 10−14, _U_ = 3497.5) and the MS proteome (_p_ < 10−15, _U_ = 135195.5), whilst the As-set has a higher CAI than the MS proteome (_p_ < 10−15, _U_ = 146964.5). (C) Molecular weight. The sizes of the proteins in each set are displayed. Proteins in the P-set are significantly smaller than those in the MS proteome (median sizes 28 and 53 kDa respectively; _p_ = 10−10, _U_ = 4685) and the As-set (median 58 kDa; _p_ = 10−9, _U_ = 11,649). The slight difference in medians between the As-set and the MS proteome is not considered significant. (D) Isoelectric point. The pI values for each data-set are shown. Both the As-set (median pI 6.2) and the P-set (median pI 10.3) are distinct from the MS proteome (median pI 6.8), but in different directions. The As- and P-sets are significantly different from each other (_p_ = 10−11, _U_ = 4057.5). The MS proteome has a slightly higher pI value than the As-set (_p_ = 0.001, _U_ = 85,761) and a significantly lower pI value than the P-set (_p_ = 10−12, _U_ = 113744.5). (E) Hydrophobicity. The GRAVY scores of each data-set are shown. The As-set has a median GRAVY score of −0.338, which is more hydrophobic than the MS proteome (−0.385; _p_ = 0.006, _U_ = 108,718) but similar to the P-set (−0.334). (F) Secondary structure. Proteins in the As- and P-sets do not show any significant differences in predicted α-helix content, while they have significantly higher α-helix content than the MS proteome (_p_ = 0.0017, _U_ = 134,098 for proteome vs. As-set; _p_ = 0.0011, _U_ = 109,179 for proteome vs. P-set). Proteins in the P-set have a significantly higher β-sheet content than proteins in the As-set (_p_ = 0.0015, _U_ = 10,036) and the MS proteome (_p_ = 4×10−4, _U_ = 110,809.5). (G) Intrinsic disorder. A fold-index was calculated where positive values represent proteins likely to be folded, and negative values represent proteins likely to be intrinsically disordered. The As-set (median fold-index 0.12) has a significantly lower proportion of intrinsically disordered proteins than the MS proteome (median fold-index 0.09; _p_ = 0.004, _U_ = 111,594), whereas the P-set has an insignificant increase in disordered proteins. (H) Protein half-lives. The half-lives for proteins in each data-set under non-stress conditions are shown. Proteins in the As- and P-sets have similar half-lives with medians of 67.5 and 84.0 minutes, respectively. These are significantly higher than for proteins in the MS proteome with a median of 53.0 minutes (_p_ = 0.0021, _U_ = 51,996 and _p_ = 0.0012, _U_ = 32805.5, respectively). Stable proteins without a measurable half-life were removed from the analysis. Outliers (> 3rd quartile + 1.5 × IQR or < 1st quartile − 1.5 × IQR) are excluded from all boxplots.
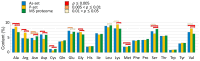
Fig. 3.. Amino acid composition of aggregation-prone proteins.
The relative amino acid composition of aggregated proteins is shown. Relative content is shown for each data-set and the _p_-values for significant differences between the sets are indicated with coloured bars. No bar indicates p > 0.05.
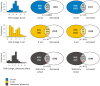
Fig. 4.. Proteins are susceptible for aggregation during translation/folding.
(A) Translation rate. Estimated translation rates per protein species are shown. Proteins in the P-set have a significantly higher translation rate (median 1.6 sec−1 per protein species) than proteins in the As-set (0.36 sec−1 per species, p = 9×10−11, U = 3649) and the MS proteome (0.19 sec−1 per species, p = 10−30, U = 19,451), while proteins in the As-set have higher translation rates than the MS proteome (p = 10−13, U = 49,020). Outliers are not shown. (B) Co-translational folding. Bars indicate the proportion of proteins in the sets that are co-translational substrates of Ssb2p. Both the As-set (68% of proteins, p = 0.005; Fisher's exact test) and the P-set (78%, p < 1×10−6) have significantly more interactions with Ssb2p than the MS proteome (55%). (C) Aggregation in SSBΔ cells. Bars show the proportion of proteins that aggregate in cells lacking Ssb1p and Ssb2p (SSBΔ). 56% of the As-set, 83% of the P-set and 40% of the MS proteome aggregate in SSBΔ cells and all differences are significant (As-set vs. MS proteome: p = 4×10−4, P-set vs. MS proteome: p < 10−15, As-set vs. P-set: p = 2×10−6; Fisher's exact test).
Fig. 5.. Arsenite toxicity mechanisms.
(A) Proportion of proteins containing cysteine clusters (CC, CxC, CxxC or CxxxC). Proteins in the As-set have a similar amount of clusters as the MS proteome, whilst the P-set stands out as significantly lower in cysteine clusters (p = 4×10−4, Fisher's exact test). (B) Occurrence of surface-exposed cysteine residues. Proteins in the As-set have a significantly higher proportion of surface-exposed redox reactive cysteines than the genome: 16% of the proteins in the As-set have at least one surface-exposed cysteine in the native fold, compared to 2% of the genome (p = 1.8×10−13; binomial test). 13% of the proteins in the P-set have at least one surface-exposed cysteine (p = 4.5×10−8). Proteins in the As-set and P-set do not differ significantly from each other (p = 0.5, G = 0.40; G-test of independence without continuity correction). (C) Number of chaperone interactions per protein. All differences are significant: As-set vs. P-set, p = 0.05; As-set vs. MS proteome, p = 10−20; P-set vs. MS proteome, p = 3×10−6; Student's t-test. Error bars represent S.D. (D) Overlap between aggregated proteins and arsenite-sensitive mutants. 90 deletion mutants were shown to be arsenite-sensitive in three independent studies (Thorsen et al., 2009). These protective proteins are not significantly overrepresented in the As-set or P-set (p = 0.8, 106 permutations). (E) Synthetic sick interactions (SSI) between As- or P-set and As-sensitive deletion mutants. Genes that have SSI with the As-set and with As-sensitive deletion mutants, as well as genes that have SSI with the P-set and with As-sensitive deletion mutants were extracted. Most significant functional enrichments compared to genome content are shown (genome = 1). (F) Physical interactions. Proteins in the As-set have on average 43 physical interactions, compared to proteins in the MS proteome that have 28 physical interactions (p = 0.00034). Proteins in the P-set have on average 80.5 physical interactions (p < 10−6).
Fig. 6.. Expression of aggregation-prone proteins is decreased during arsenite exposure.
The histograms show the relative change in expression for proteins in the As-set, P-set and stationary phase aggregates in response to 1.0 mM arsenite (Thorsen et al., 2007). The As-set and P-set are notably shifted toward decreased expression. The Venn diagrams show the overlap between the data-sets and proteins with at least a 2-fold change in gene expression following arsenite exposure. Relative numbers give the proportion of proteins that are differentially expressed or that show a less than 2-fold change in gene expression. Numbers in parentheses give the absolute number of proteins in the intersection and the representation factor, i.e. observed/expected, is shown in red. _p_-values were calculated with hypergeometric tests using the MS proteome as background. The As-set is shifted toward lower expression, having 50% more proteins with ≥ 2-fold lower expression than expected (p = 0.0003, hypergeometric test). Proteins in the P-set show a decreased expression with 69% having ≥ 2-fold decreased expression, which is 2.6 times more than expected as compared to the MS proteome (p = 10−23), and 3% having a ≥ 2-fold increased expression, which is only one third of what is expected. Proteins that aggregate in stationary phase show no consistent trend, with proteins being both up- and down-regulated during arsenite exposure.
Similar articles
- Arsenite interferes with protein folding and triggers formation of protein aggregates in yeast.
Jacobson T, Navarrete C, Sharma SK, Sideri TC, Ibstedt S, Priya S, Grant CM, Christen P, Goloubinoff P, Tamás MJ. Jacobson T, et al. J Cell Sci. 2012 Nov 1;125(Pt 21):5073-83. doi: 10.1242/jcs.107029. Epub 2012 Sep 3. J Cell Sci. 2012. PMID: 22946053 - Genome-wide imaging screen uncovers molecular determinants of arsenite-induced protein aggregation and toxicity.
Andersson S, Romero A, Rodrigues JI, Hua S, Hao X, Jacobson T, Karl V, Becker N, Ashouri A, Rauch S, Nyström T, Liu B, Tamás MJ. Andersson S, et al. J Cell Sci. 2021 Jun 1;134(11):jcs258338. doi: 10.1242/jcs.258338. Epub 2021 Jun 4. J Cell Sci. 2021. PMID: 34085697 Free PMC article. - Dual role of ribosome-associated chaperones in prion formation and propagation.
Chernoff YO, Kiktev DA. Chernoff YO, et al. Curr Genet. 2016 Nov;62(4):677-685. doi: 10.1007/s00294-016-0586-2. Epub 2016 Mar 11. Curr Genet. 2016. PMID: 26968706 Review. - Distinct stress conditions result in aggregation of proteins with similar properties.
Weids AJ, Ibstedt S, Tamás MJ, Grant CM. Weids AJ, et al. Sci Rep. 2016 Apr 18;6:24554. doi: 10.1038/srep24554. Sci Rep. 2016. PMID: 27086931 Free PMC article. - Challenging Proteostasis: Role of the Chaperone Network to Control Aggregation-Prone Proteins in Human Disease.
Sinnige T, Yu A, Morimoto RI. Sinnige T, et al. Adv Exp Med Biol. 2020;1243:53-68. doi: 10.1007/978-3-030-40204-4_4. Adv Exp Med Biol. 2020. PMID: 32297211 Free PMC article. Review.
Cited by
- Methionine Sulfoxide Reductases Suppress the Formation of the [PSI+] Prion and Protein Aggregation in Yeast.
Schepers J, Carter Z, Kritsiligkou P, Grant CM. Schepers J, et al. Antioxidants (Basel). 2023 Feb 7;12(2):401. doi: 10.3390/antiox12020401. Antioxidants (Basel). 2023. PMID: 36829961 Free PMC article. - Proteome-wide observation of the phenomenon of life on the edge of solubility.
Vecchi G, Sormanni P, Mannini B, Vandelli A, Tartaglia GG, Dobson CM, Hartl FU, Vendruscolo M. Vecchi G, et al. Proc Natl Acad Sci U S A. 2020 Jan 14;117(2):1015-1020. doi: 10.1073/pnas.1910444117. Epub 2019 Dec 31. Proc Natl Acad Sci U S A. 2020. PMID: 31892536 Free PMC article. - Native Fold Delay and its implications for co-translational chaperone binding and protein aggregation.
Duran-Romaña R, Houben B, Migens PF, Zhang Y, Rousseau F, Schymkowitz J. Duran-Romaña R, et al. Nat Commun. 2025 Feb 15;16(1):1673. doi: 10.1038/s41467-025-57033-z. Nat Commun. 2025. PMID: 39955309 Free PMC article. - The non-stop decay mRNA surveillance pathway is required for oxidative stress tolerance.
Jamar NH, Kritsiligkou P, Grant CM. Jamar NH, et al. Nucleic Acids Res. 2017 Jun 20;45(11):6881-6893. doi: 10.1093/nar/gkx306. Nucleic Acids Res. 2017. PMID: 28472342 Free PMC article. - Aggregation and disaggregation features of the human proteome.
Määttä TA, Rettel M, Sridharan S, Helm D, Kurzawa N, Stein F, Savitski MM. Määttä TA, et al. Mol Syst Biol. 2020 Oct;16(10):e9500. doi: 10.15252/msb.20209500. Mol Syst Biol. 2020. PMID: 33022891 Free PMC article.
References
- Basso M., Samengo G., Nardo G., Massignan T., D'Alessandro G., Tartari S., Cantoni L., Marino M., Cheroni C., De Biasi S. et al. (2009). Characterization of detergent-insoluble proteins in ALS indicates a causal link between nitrative stress and aggregation in pathogenesis. PLoS ONE 4, e8130 10.1371/journal.pone.0008130 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases