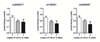Epistasis between polymorphisms in COMT, ESR1, and GCH1 influences COMT enzyme activity and pain - PubMed (original) (raw)
Epistasis between polymorphisms in COMT, ESR1, and GCH1 influences COMT enzyme activity and pain
Shad B Smith et al. Pain. 2014 Nov.
Abstract
Abnormalities in the enzymatic activity of catechol-O-methyltransferase (COMT) contribute to chronic pain conditions, such as temporomandibular disorders (TMD). Thus, we sought to determine the effects of polymorphisms in COMT and functionally related pain genes in the COMT pathway (estrogen receptor 1 [ESR1], guanosine-5-triphosphate cyclohydrolase 1 [GCH1], methylenetetrahydrofolate reductase [MTHFR]) on COMT enzymatic activity, musculoskeletal pain, and pain-related intermediate phenotypes among TMD cases and healthy control subjects. Results show that the COMT rs4680 (val(158)met) polymorphism is most strongly associated with outcome measures, such that individuals with the minor A allele (met) exhibit reduced COMT activity, increased TMD risk, and increased musculoskeletal pain. Epistatic interactions were observed between the COMT rs4680 polymorphism and polymorphisms in GCH1 and ESR1. Among individuals with the COMT met allele, those with 2 copies of the GCH1 rs10483639 minor G allele exhibit normalized COMT activity and increased mechanical pain thresholds. Among individuals with the COMT val allele, those with 2 copies of the ESR1 rs3020377 minor A allele exhibit reduced COMT activity, increased bodily pain, and poorer self-reported health. These data reveal that the GCH1 minor G allele confers a protective advantage among met carriers, whereas the ESR1 minor A allele is disadvantageous among val carriers. Furthermore, these data suggest that the ability to predict the downstream effects of genetic variation on COMT activity is critically important to understanding the molecular basis of chronic pain conditions.
Keywords: Catecholamines; Fibromyalgia; Gene expression; Gene–gene interaction; Temporomandibular disorders.
Copyright © 2014 International Association for the Study of Pain. Published by Elsevier B.V. All rights reserved.
Figures
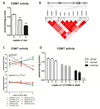
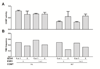
Fig. 1. COMT genotype effects COMT activity
A. COMT val158met genotype effects COMT activity, such that individuals with 1 or 2 copies of the met allele exhibit reduced activity. Data represent mean ± s.e.m. ***p < 0.0001. B. Linkage disequilibrium plot of the genotyped SNPs in the COMT gene locus. The gene diagram indicates the location of each SNP with respect to exons (boxes) and introns of the gene. The color of each box in the LD plot depicts the strength of LD between aligned SNPs according to D’ (darker red indicates stronger LD), and the number in the box is the corresponding r2 value. C. COMT activity levels of each genotype are shown for all COMT SNPs tested. Symbols and lines are colored green if the respective minor allele is associated with increased COMT activity, and red with decreased COMT activity; the rs4680 val158met is shown in black. In the top panel genotype effects of each SNP are unadjusted; in the bottom panel each SNP effect is adjusted for val158met genotype. D. COMT activity level of each genotype of rs737865, stratified by val158met genotype, shows that the minor G allele of this SNP is associated with decreased COMT activity after the confounding effect of val158met is removed.
Fig. 2. ESR1 SNP effects on COMT activity
All three ESR1 SNPs showed significant (p<0.05) association with COMT activity using a codominant model, although the dominant model provides a better fit for the data for rs3020377. Data represent mean ± s.e.m.
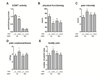
Fig. 3. COMT × ESR1 effects on COMT activity and pain phenotypes
For genotypes of the ESR1 SNP rs3020377, all carriers of the minor G allele were combined (dominant test) and stratified by carriers of COMT alleles val and met. Significance level shown is that of the test of the G allele carriers against the reference of the major allele homozygote group, within val carriers only. Among val carriers, the ESR1 rs3020377 SNP produced effects on A. COMT enzymatic activity, B. physical functioning subscale of the SF12 questionnaire, C. pain intensity rating using the Gracely scale of the SPSR, D. pain unpleasantness rating using the Gracely Pain Scale of the SPSR, and E. count of the number of bodily sites painful upon palpation by clinical examiner. For each phenotype, rs3020377 modulates the effect of the val allele, but not the met allele, of COMT. The G allele of rs3020377 decreased COMT activity and physical functioning, and increased nociceptive phenotypes of pain intensity, unpleasantness, and distribution across body sites. Data are quantitative trait means ± s.e.m. † p < 0.1 * p< 0.05 ** p<0.01.
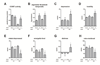
Fig. 4. COMT × GCH1 effects on COMT activity and pain phenotypes
The GCH1 SNP rs10483639 showed a recessive mode of inheritance, and was stratified by carriers of the COMT alleles val and met. Significance level shown is that of the test of the C allele homozygotes against the reference of the major allele carriers, within met carriers only. Among met carriers, the GCH1 rs10483639 SNP produced effects on A. COMT enzymatic activity, B. algometer pressure at which pain is detected, measured at the temporalis muscle, C. depression subscale of the SCL90 questionnaire, D. hostility subscale of the SCL90, E. elated-depressed axis of the POMS, F. energetic-tired axis of the POMS. G. distress subscale of the PSS, and H. dysfunction in daily activities due to emotional factors, measured by the SF12 questionnaire. Carrying two copies of the rs10483639 C allele raised COMT activity in met carriers to the level of val carriers, but had no effect in the high-activity val carriers. The C allele was associated with higher algometer threshold in both val and met carriers, but was associated with more negative psychological characteristics in met carriers only. Data are quantitative trait means ± s.e.m. * p< 0.05.
Fig. 5. Three-way interaction between COMT, ESR1, and GCH1 SNPs on COMT activity and TMD risk
A. COMT enzyme activity (means ± s.e.m.) and B. TMD frequency is plotted for each genotype. The GCH1 rs10483639 C allele increases COMT activity and reduces TMD risk among met carriers, while the ESR1 rs3020377 G allele decreases COMT activity and increases TMD risk among val carriers with only 0 or 1 copy of the GCH1 rs10483639 C allele.
Similar articles
- Association analysis of the catechol-O-methyltransferase /methylenetetrahydrofolate reductase genes and cognition in late-onset depression.
Wang X, Wang Z, Wu Y, Yuan Y, Hou Z, Hou G. Wang X, et al. Psychiatry Clin Neurosci. 2014 May;68(5):344-52. doi: 10.1111/pcn.12133. Epub 2013 Dec 25. Psychiatry Clin Neurosci. 2014. PMID: 24373005 - The MAOA, COMT, MTHFR and ESR1 gene polymorphisms are associated with the risk of depression in menopausal women.
Różycka A, Słopień R, Słopień A, Dorszewska J, Seremak-Mrozikiewicz A, Lianeri M, Maciukiewicz M, Warenik-Szymankiewicz A, Grzelak T, Kurzawińska G, Drews K, Klejewski A, Jagodziński PP. Różycka A, et al. Maturitas. 2016 Feb;84:42-54. doi: 10.1016/j.maturitas.2015.10.011. Epub 2015 Oct 30. Maturitas. 2016. PMID: 26620113 - Polymorphisms of Nav1.6 sodium channel, Brain-derived Neurotrophic Factor, Catechol-O-methyltransferase and Guanosine Triphosphate Cyclohydrolase 1 genes in trigeminal neuralgia.
Romero J, Costa GMF, Rocha LPC, Siqueira S, Moreira PR, Almeida-Leite CM. Romero J, et al. Clin Neurol Neurosurg. 2021 Sep;208:106880. doi: 10.1016/j.clineuro.2021.106880. Epub 2021 Aug 8. Clin Neurol Neurosurg. 2021. PMID: 34418703 - Catechol-O-methyltransferase gene polymorphism and chronic human pain: a systematic review and meta-analysis.
Tammimäki A, Männistö PT. Tammimäki A, et al. Pharmacogenet Genomics. 2012 Sep;22(9):673-91. doi: 10.1097/FPC.0b013e3283560c46. Pharmacogenet Genomics. 2012. PMID: 22722321 Review. - Are catechol-O-methyltransferase gene polymorphisms genetic markers for pain sensitivity after all? - A review and meta-analysis.
Vetterlein A, Monzel M, Reuter M. Vetterlein A, et al. Neurosci Biobehav Rev. 2023 May;148:105112. doi: 10.1016/j.neubiorev.2023.105112. Epub 2023 Feb 24. Neurosci Biobehav Rev. 2023. PMID: 36842714 Review.
Cited by
- Low catechol-O-methyltransferase and stress potentiate functional pain and depressive behavior, especially in female mice.
Zhang X, Kanter K, Chen J, Kim S, Wang Y, Adeyemi C, O'Buckley SC, Nackley AG. Zhang X, et al. Pain. 2020 Feb;161(2):446-458. doi: 10.1097/j.pain.0000000000001734. Pain. 2020. PMID: 31972854 Free PMC article. - Persistent Catechol-O-methyltransferase-dependent Pain Is Initiated by Peripheral β-Adrenergic Receptors.
Ciszek BP, O'Buckley SC, Nackley AG. Ciszek BP, et al. Anesthesiology. 2016 May;124(5):1122-35. doi: 10.1097/ALN.0000000000001070. Anesthesiology. 2016. PMID: 26950706 Free PMC article. - Pain, Genes, and Function in the Post-Hip Fracture Period.
Resnick B, Klinedinst NJ, Yerges-Armstrong L, Magaziner J, Orwig D, Hochberg MC, Gruber-Baldini AL, Hicks GE, Dorsey SG. Resnick B, et al. Pain Manag Nurs. 2016 Jun;17(3):181-96. doi: 10.1016/j.pmn.2016.03.003. Pain Manag Nurs. 2016. PMID: 27283266 Free PMC article. - Biomarkers in Temporomandibular Disorder and Trigeminal Neuralgia: A Conceptual Framework for Understanding Chronic Pain.
Doshi TL, Nixdorf DR, Campbell CM, Raja SN. Doshi TL, et al. Can J Pain. 2020;4(1):1-18. doi: 10.1080/24740527.2019.1709163. Epub 2020 Jan 23. Can J Pain. 2020. PMID: 32923920 Free PMC article. - Painful Temporomandibular Joint Clicking: Genetic Point of View.
Poluha RL, Carvalho Soares FF, Furquim BD, De la Torre Canales G, Sales Pinto Fiamengui LM, Bonjardim LR, Rodrigues Conti PC. Poluha RL, et al. J Oral Facial Pain Headache. 2022 Summer;36(3-4):229–235. doi: 10.11607/ofph.3115. Epub 2022 Nov 28. J Oral Facial Pain Headache. 2022. PMID: 36445911 Free PMC article.
References
- Ahlers SJ, Elens LL, van Gulik L, van Schaik RH, van Dongen EP, Bruins P, Tibboel D, Knibbe CA. The Val158Met polymorphism of the COMT gene is associated with increased pain sensitivity in morphine-treated patients undergoing a painful procedure after cardiac surgery. Br J Clin Pharmacol. 2013;75(6):1506–1515. - PMC - PubMed
- Antoniades C, Shirodaria C, Van Assche T, Cunnington C, Tegeder I, Lotsch J, Guzik TJ, Leeson P, Diesch J, Tousoulis D, Stefanadis C, Costigan M, Woolf CJ, Alp NJ, Channon KM. GCH1 haplotype determines vascular and plasma biopterin availability in coronary artery disease effects on vascular superoxide production and endothelial function. J Am Coll Cardiol. 2008;52(2):158–165. - PMC - PubMed
- Barbosa FR, Matsuda JB, Mazucato M, de Castro Franca S, Zingaretti SM, da Silva LM, Martinez-Rossi NM, Junior MF, Marins M, Fachin AL. Influence of catechol-O-methyltransferase (COMT) gene polymorphisms in pain sensibility of Brazilian fibromialgia patients. Rheumatol Int. 2012;32(2):427–430. - PubMed
- Barnett JH, Heron J, Ring SM, Golding J, Goldman D, Xu K, Jones PB. Gender-specific effects of the catechol-O-methyltransferase Val108/158Met polymorphism on cognitive function in children. Am J Psychiatry. 2007;164(1):142–149. - PubMed
- Belfer I, Schreiber KL, Shaffer JR, Shnol H, Blaney K, Morando A, Englert D, Greco C, Brufsky A, Ahrendt G, Kehlet H, Edwards RR, Bovbjerg DH. Persistent postmastectomy pain in breast cancer survivors: analysis of clinical, demographic, and psychosocial factors. J Pain. 2013;14(10):1185–1195. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- K12 HD052191/HD/NICHD NIH HHS/United States
- L30 NS075905/NS/NINDS NIH HHS/United States
- P01 NS045685/NS/NINDS NIH HHS/United States
- R01 NS072205/NS/NINDS NIH HHS/United States
- L30 DE017059/DE/NIDCR NIH HHS/United States
- K12 DE022793/DE/NIDCR NIH HHS/United States
- U01 DE017018/DE/NIDCR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous