Endothelial podosome rosettes regulate vascular branching in tumour angiogenesis - PubMed (original) (raw)
. 2014 Oct;16(10):931-41, 1-8.
doi: 10.1038/ncb3036. Epub 2014 Sep 14.
Giulia Chiaverina 2, Paolo Armando Gagliardi 2, Laura di Blasio 2, Alberto Puliafito 2, Claire Bouvard 3, Roberto Sessa 2, Guido Tarone 4, Lydia Sorokin 5, Dominique Helley 6, Rakesh K Jain 7, Guido Serini 2, Federico Bussolino 2, Luca Primo 2
Affiliations
- PMID: 25218639
- PMCID: PMC4564017
- DOI: 10.1038/ncb3036
Endothelial podosome rosettes regulate vascular branching in tumour angiogenesis
Giorgio Seano et al. Nat Cell Biol. 2014 Oct.
Abstract
The mechanism by which angiogenic endothelial cells break the physical barrier of the vascular basement membrane and consequently sprout to form new vessels in mature tissues is unclear. Here, we show that the angiogenic endothelium is characterized by the presence of functional podosome rosettes. These extracellular-matrix-degrading and adhesive structures are precursors of de novo branching points and represent a key feature in the formation of new blood vessels. VEGF-A stimulation induces the formation of endothelial podosome rosettes by upregulating integrin α6β1. In contrast, the binding of α6β1 integrin to the laminin of the vascular basement membrane impairs the formation of podosome rosettes by restricting α6β1 integrin to focal adhesions and hampering its translocation to podosomes. Using an ex vivo sprouting angiogenesis assay, transgenic and knockout mouse models and human tumour sample analysis, we provide evidence that endothelial podosome rosettes control blood vessel branching and are critical regulators of pathological angiogenesis.
Figures
Figure 1
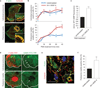
VEGF-A induces endothelial podosome rosettes. (a) Immunostained representative ECs treated with PMA for 30 min. Insets, zoom of the same micrograph. Scale bars, 10 µm. (b) ECs—incubated for 24 h in M199 10% FCS (unstimulated) or in M199 10% FCS plus 30 ng ml−1 of VEGF-A (24 h VEGF-A)—were treated for 30 min with PMA. We calculated the percentage of individual-podosome- and podosome-rosette-positive ECs, treated with PMA for the indicated times. Mean ± s.e.m. of n = 3 independent experiments in which 250 cells were analysed per experimental point. (c) Cytofluorimetric analysis of the active form of MT1-MMP. ECs treated with PMA for 30 min. Normalized mean ± s.e.m. of n = 3 independent experiments in which 9 × 104 cells were analysed per experimental point. (d) Gelatin degradation assay by TIRF micrographs. ECs were seeded on FITC-conjugated gelatin, PMA-treated and then stained with phalloidin. The white dashed line is the outline of the cell boundary and is traced here as a guide to the eye. White arrows indicate the areas in which gelatin was degraded by individual podosomes or podosome rosettes. Scale bars, 10 µm. (e,f) Ex vivo VEGF-A stimulation induces podosome rosettes in aortic vessels. Aortic explants were incubated for 48 h in M199 10% FCS (unstimulated) or M199 10% FCS with 30 ng ml−1 of VEGF-A (48 h VEGF-A). (e) Immunostaining of a representative 48 h VEGF-A-stimulated aortic explant. Inset, a podosome rosette. Scale bar, 20 µm. Single-channel images are in Supplementary Fig. 1e. (f) Graph showing the percentage of podosome-rosette-positive ECs in the endothelial layer of aortic explants. Mean ± s.e.m. of _n_=3 independent experiments in which 511 nuclei were analysed per experimental point. (b,c,f) Statistical significance was calculated using an unpaired non-parametric Mann-Whitney test (**P<0.01 versus unstimulated.).
Figure 2
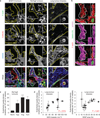
Tumour angiogenic vessels are characterized by high levels of endothelial podosome rosettes. (a) Confocal imaging stacks of representative vessels in subcutaneous B16F10 melanoma, in angiogenic islets of transgenic RipTag2 mice or human samples of lung tumours. _xyz_-section of immunostaining for primary antibodies as indicated. Vessels are delimited by dashed lines; arrows indicate podosome rosettes. Inset, the podosome rosette. Schematization and 3D rendering in Supplementary Fig. 2a,b. Scale bars, 10 µm. (b) In situ zymography in RipTag2 angiogenic islets. _xyz_-section of staining for primary antibodies as indicated and gelatin-DQ (dye-quenched), showing the degradated gelatin. Vessels are delimited by white dashed lines; white arrows indicate podosome rosettes. Inset, the podosome rosette. 3D rendering in Supplementary Fig. 2e. Scale bar, 10µm. (c) Graph shows the density of podosome rosettes in vessels of RipTag2 tumour mouse stages. Vessel regions of interest were determined with laminin staining. Mean ± s.e.m. of n = 3 mice, 5 fields per tumour stage. Statistical significance was calculated using a one-way ANOVA test followed by Bonferroni-adjusted post hoc _t_-tests (**P<0.01 versus normal islets; ***P < 0.001 versus normal islets). (d) Scatter plots of the density of endothelial podosome rosettes versus microvessel density (MVD)-CD31 (_r_2=0.49, P = 0.016) and VEGF area fraction in biopsy samples of lung tumours (_r_2=0.46, P = 0.021). Mean ± s.e.m. of n = 3 different vessels per biopsy for rosette density and n = 20 fields per slide for MVD and VEGF. Statistical significance was calculated using a Pearson correlation test. Representative images are shown in Supplementary Fig. 3c.
Figure 3
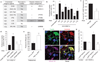
Integrin α6 is essential for VEGF-induced endothelial podosome rosette formation and function. (a) Table of integrin recruitment in endothelial podosome rosettes and functional blocking treatment. The qualitative analysis of rosette blockade is based on podosome-rosette-positive cells percentages in comparison with aspecific IgG treatment. Confocal micrographs of integrin recruitment are shown in Supplementary Fig. 4a. (b) Graph showing the percentages of podosome-rosette-positive ECs, stimulated as indicated and treated with aspecific IgG or anti-integrin blocking antibodies 2 h before PMA treatment. ECs were treated with IgG or anti-integrin blocking antibody (20 µg ml−1) during cell adhesion and then stimulated with PMA for 30 min. Mean ± s.e.m. of n = 3 independent experiments in which 250 cells were analysed per experimental point. Statistical significance was calculated using a one-way ANOVA test followed by Bonferroni-adjusted post hoc t_-tests (°°°_P<0.001 versus unstimulated; *P<0.05 versus 24 h VEGF-A IgG; ***P<0.001 versus 24 h VEGF-A IgG). (c) Cytofluorimetric analysis of active MT1-MMP in VEGF-A-stimulated ECs, treated with rat IgG or anti-α6 blocking antibody and then stimulated with PMA for 30 min. Normalized mean ± s.e.m. of n = 3 independent experiments in which 9 × 104 cells were analysed per experimental point. Statistical significance was calculated using an unpaired non-parametric Mann-Whitney test (**P<0.01 versus rat IgG). (d) Graph showing the percentages of podosome-rosette-positive ECs, transduced with scramble (SCRL) shRNA or shRNA against integrin α6 (ITGA6 shRNA4 and ITGA6 shRNA5). Membrane integrin α6 levels in transduced ECs are shown in Supplementary Fig. 4d. Mean ± s.e.m. of n = 3 independent experiments in which 250 cells were analysed per experimental point. Statistical significance was calculated using a one-way ANOVA test followed by Bonferroni-adjusted post hoc t_-tests (°°_P<0.01 versus Unstim SCRL shRNA; **P<0.01 versus SCRL shRNA). (e) Lifespan of the podosomes that form endothelial rosettes in α6-GFP- and LifeAct-RFP-transduced ECs. Graph shows the lifespan in minutes of podosomes with low or high levels of integrin α6 detected with TIRF microscopy (90 nm of depth) in VEGF-stimulated ECs. Mean ± s.e.m. of n = 230 podosomes from 3 different cells. Statistical significance was calculated using an unpaired non-parametric Mann-Whitney test (**P< 0.01 versus low ITGA6). (f) Endothelial layer of a 48 h VEGF-A-stimulated aortic explant immunostained by the indicated antibodies and nuclear-stained by DAPI (blue). Inset, a podosome rosette in the basal side of the endothelial layer. Scale bar, 20 µm. (g) VEGF-A stimulation in aortic explants of Tie2-dependent α6 null mice. Aortic explants from WT (α6_fl/fl-Tie2Cre_−) or endothelial α6 null (α6_fl/fl-Tie2Cre+) mice were incubated for 48 h in M199 10% FCS (unstim) or M199 10% FCS with 30 ng ml−1 of VEGF-A (48 h VEGF-A). Mean ± s.e.m. of n = 3 independent experiments in which 1,250 nuclei were analysed per experimental point. Statistical significance was calculated using two-way ANOVA test followed by Bonferroni-adjusted post hoc t_-tests (°_P<0.05 versus unstim α6_fl/fl-Tie2Cre−; ***P<0.001 versus 48 h VEGF-A α6_fl/fl-Tie2Cre−_).
Figure 4
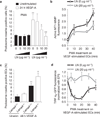
Laminin impairs podosome rosette formation. (a) Graph showing the percentages of podosome-rosette-positive ECs, stimulated as indicated, seeded on gelatin-coated coverslips with the indicated addition of laminin. Percentages of individual-podosome-positive cells are in Supplementary Fig. 6a. Mean ± s.e.m. of n = 3 independent experiments in which 260 cells were analysed per experimental point. Statistical significance was calculated using a two-way ANOVA test followed by Bonferroni-adjusted post hoc _t_-tests (°° P<0.01 versus unstimulated; *P<0.05 versus LN (0 µg ml −1); **P<0.01 versus LN (0 µg ml−1)). (b) Cytofluorimetric analysis of active MT1-MMP in VEGF-stimulated ECs, seeded on a gelatin coating with the indicated addition of laminin. Normalized mean ± s.e.m. of n = 3 independent experiments in which 105 cells were analysed per experimental point. Statistical significance was calculated using a two-way ANOVA test followed by Bonferroni-adjusted post hoc _t_-tests (*P<0.05 versus LN (0 µg ml−1); **P < 0.01 versus LN (0 g ml−1)). (c) VEGF-A stimulation in aortic explants of laminin α4 null mice. Aortic explants from Lama4+/+ or _Lama4_−/− mice were incubated for 48 h in M199 10% FCS (unstim) or M199 10% FCS with 30 ng ml−1 of VEGF-A (48 h VEGF-A). Mean ± s.e.m. of n = 3 independent experiments in which 550 nuclei were analysed per experimental point. Statistical significance was calculated using a two-way ANOVA test followed by Bonferroni-adjusted post hoc _t_-tests (°° P < 0.05 versus unstimulated Lama4+/+; *P<0.05 versus 48 h VEGF-A Lama4+/+). (d) Integrin α6 membrane localization is modulated by laminin in the substratum. The graph shows the ratio of α6-GFP fluorescence in the membrane (TIRF microscopy with <90 nm of deepness) and in the whole cell (epifluorescence, EPI) in the indicated periods of PMA treatment; mean ± s.e.m. of n = 30 cells from 3 independent experiments.
Figure 5
α6 integrin–laminin binding in FAs slows down α6 integrin translocation to podosome rosettes. (a) Time-lapse TIRF microscopy of vinculin–RFP-transfected ECs during PMA treatment. Insets, podosome rosettes indicated by arrows. For complete video, see Supplementary Video 3. Scale bar, 20 µm. (b) Graph showing the percentages of podosome-rosette-positive ECs, treated as indicated. CHX: cycloheximide; Noco: nocodazole; PQ: primaquine; NocoWO: nocodazole washout. Mean ± s.e.m. of n = 3 independent experiments in which 200 cells were analysed per experimental point. Statistical significance was calculated using a one-way ANOVA test followed by Bonferroni-adjusted post hoc t_-tests ***P<0.05 versus PMA treated; ***P< 0.001 versus PMA treated; °°°_P< 0.001 versus NocoWO+PMA). (c) Graph showing the percentages of podosome-rosette-positive ECs, seeded on gelatin-coated coverslips with the indicated laminin addition and PMA-stimulated with or without nocodazole washout. Percentages of individual-podosome-positive cells are in Supplementary Fig. 6g. Mean ± s.e.m. of _n_=3 independent experiments in which 230 cells were analysed per experimental point. Statistical significance was calculated using a two-way ANOVA test followed by Bonferroni-adjusted post hoc t_-tests (°_P<0.05 versus no inhib LN (0 µg ml−1); *P< 0.05 versus no inhib LN (20 µg ml−1)). (d) Graph showing the percentages of podosome-rosette-positive ECs, seeded on gelatin-coated coverslips with the indicated addition of laminin. ECs were transduced as indicated. Membrane integrin α6 levels in transduced ECs are shown in Supplementary Fig. 4d. Percentages of individual-podosome-positive cells are in Supplementary Fig. 6h. Mean ± s.e.m. of _n_= 3 independent experiments in which 320 cells were analysed per experimental point. Statistical significance was calculated using one-way ANOVA test followed by Bonferroni-adjusted post hoc t_-tests (*P<0.05 versus empty vector L(10 µg ml−1); °_P < 0.05 versus empty vector L(20 µg ml−1). (e) Graph showing the percentages of podosome-rosette-positive ECs, seeded on gelatin-coated coverslips with 20 µg ml−1 laminin. ECs were transduced as indicated and stimulated or not with VEGF-A for 24 h. Mean ± s.e.m. of _n_= 3 independent experiments in which 420 cells were analysed per experimental point. Statistical significance was calculated using a two-way ANOVA test followed by Bonferroni-adjusted post hoc t_-tests (*P<0.05 versus its corresponding empty vector; °_P < 0.05 versus empty vector L-VEGF-A−; # P < 0.05 versus empty vector L-VEGF-A+).
Figure 6
Endothelial podosome rosettes precede vessel branching from a pre-existing vessel. (a) Confocal image stacks of representative angiogenic outgrowths from 7-day mARs into collagen gel (left panel). Scale bar, 50 µm. Right panel, xyz_-section of the white dotted square in the left panel. Scale bar, 10µm. (b) 3D isosurface rendering of endothelial rosettes in angiogenic outgrowths. Angiogenic outgrowths (grey) and podosome-rosettes (red) were recognized with co-localization of F-actin/cortactin staining (indicated by red arrows) as detailed in the Supplementary Methods. The white asterisk indicates the tip-cell nucleus. Inset, a representative endothelial rosette. For complete video, see Supplementary Video 4. Scale bar, 30 µm. (c,d) Time-lapse multiphoton microscopy of angiogenic outgrowths from LifeAct–EGFP mARs. For complete video, see Supplementary Videos 5 and 6. The podosome rosette and cell protrusion are indicated by white arrows. Scale bar, 20 µm in c and 50µm in d. (e) Branching density—number of branching points divided by vascular area—of angiogenic outgrowths from WT (α6_fl/fl-Tie2Cre−) or endothelial α6 null (α6_fl/fl-Tie2Cre+) mARs. Dynamical analysis of branching shown in Supplementary Video 7. Mean ± s.e.m. of n = 18 mARs, 3 mARs per mouse from 6 mice. Statistical significance was calculated using a two-way ANOVA test followed by Bonferroni-adjusted post hoc t_-tests (*P < 0.05 versus α6_fl/fl-Tie2Cre−_). (f) Branching density of angiogenic outgrowths from laminin α4 null mARs into collagen with or without laminin addition. Dynamical analysis of _Lama4_−/− branching is shown in Supplementary Video 8 and laminin in gel in Supplementary Video 9. Mean ± s.e.m. of n = 8 mARs, 2 mARs per mouse from 4 mice. Statistical significance was calculated using a two-way ANOVA test followed by Bonferroni-adjusted post hoc t_-tests (***P<0.001 versus LN (0 µg ml−1) WT; °_P<0.05 versus LN (0 µg ml −1) _Lama4_−/−).
Figure 7
In vivo blocking of integrin α6 impairs endothelial podosome rosette formation and reduces vessel branching in tumours. (a) Rapid accumulation of anti-α6 integrin antibody into endothelial podosome rosettes of RipTag2 tumour vessels. xyz_-sections of confocal micrographs of the distribution of immunoreactivity in RipTag2 tumours 10 min after intravenous injection of 25 µg of anti-α6 integrin antibody. Vessels are delimited by white dotted lines; white arrows indicate the podosome rosette. Inset, high magnification of the podosome rosette. Scale bar, 5 µm. (b) Measurements of rosette density in vessels of RipTag2 mouse tumours, treated with rat IgG or anti-α6 blocking antibody. Mean ± s.e.m. of n = 30 fields, 5 fields per pancreatic islet from 6 mice per treatment group. Statistical significance was calculated using an unpaired non-parametric Mann-Whitney test (**P<0.01 versus rat IgG.). (c) Branching density in blocking antiα6-treated RipTag2 tumours. Mean ± s.e.m. of n = 30 fields, 5 fields per mouse from 6 mice per treatment group. Statistical significance was calculated using an unpaired non-parametric Mann-Whitney test (*P<0.05 versus rat IgG). (d) Measurements of rosette density in vessels of gastrocnemius muscles from unilateral hindlimb ischaemia experiments in WT (α6_fl/fl-Tie2Cre−) or endothelial α6 null (α6_fl/fl-Tie2Cre+) mice. Mean ± s.e.m. of n = 9 fields, 3 fields per muscle from 3 mice. Statistical significance was calculated using an unpaired non-parametric Mann-Whitney test (°°_P < 0.01 versus normal α6_fl/fl-Tie2Cre_−; *P<0.05 versus α6_fl/fl-Tie2Cre_+). (e) Measurements of rosette density in vessels of subcutaneous B16-F10 tumours in WT (α6_fl/fl-Tie2Cre_−) or endothelial α6 null (α6_fl/fl-Tie2Cre+_) mice. Mean ± s.e.m. of n = 21 fields, 3 fields per tumour from 7 mice per treatment group. Statistical significance was calculated using an unpaired non-parametric Mann-Whitney test (**P< 0.01 versus α6_fl/fl-Tie2Cre_−). (f) Branching density in B16F10 melanoma subcutaneously injected in Tie2-dependent α6 KO mice. Mean ± s.e.m. of n = 42 fields, 5 fields per tumour from 7 mice per treatment group. Statistical significance was calculated using an unpaired non-parametric Mann-Whitney test (**P<0.01 versus α6_fl/fl-Tie2Cre_− mice). (g) Cartoon showing α6 integrin/laminin molecular mechanisms involved in sprouting angiogenesis. (1) Quiescent EC have low levels of α6β1 integrin, which binds vBM laminin, is recruited in FAs, and results in blood vessel stabilization. (2) When the tumour produces VEGF, the VEGF induces upregulation of the α6 integrin subunit in ECs. The increased availability of α6β1 integrin then allows the formation and stabilization of endothelial podosome rosettes and the ensuing MMP-driven degradation of ECM that, in turn, (3) allows vBM invasion by ECs and sprouting angiogenesis.
Comment in
- Angiogenesis: pushing through, branching out.
Villanueva MT. Villanueva MT. Nat Rev Cancer. 2014 Nov;14(11):704-5. doi: 10.1038/nrc3841. Epub 2014 Oct 9. Nat Rev Cancer. 2014. PMID: 25291293 No abstract available. - Cell adhesion: Winning mechanism for angiogenesis.
Minton K. Minton K. Nat Rev Mol Cell Biol. 2014 Nov;15(11):702. doi: 10.1038/nrm3893. Epub 2014 Oct 15. Nat Rev Mol Cell Biol. 2014. PMID: 25315274 No abstract available.
Similar articles
- Podosome rosettes precede vascular sprouts in tumour angiogenesis.
Warren CM, Iruela-Arispe ML. Warren CM, et al. Nat Cell Biol. 2014 Oct;16(10):928-30. doi: 10.1038/ncb3044. Nat Cell Biol. 2014. PMID: 25271481 Free PMC article. - TGFbeta-induced endothelial podosomes mediate basement membrane collagen degradation in arterial vessels.
Rottiers P, Saltel F, Daubon T, Chaigne-Delalande B, Tridon V, Billottet C, Reuzeau E, Génot E. Rottiers P, et al. J Cell Sci. 2009 Dec 1;122(Pt 23):4311-8. doi: 10.1242/jcs.057448. Epub 2009 Nov 3. J Cell Sci. 2009. PMID: 19887587 - Podosomes as novel players in endothelial biology.
Seano G, Daubon T, Génot E, Primo L. Seano G, et al. Eur J Cell Biol. 2014 Oct;93(10-12):405-12. doi: 10.1016/j.ejcb.2014.07.009. Epub 2014 Aug 7. Eur J Cell Biol. 2014. PMID: 25199436 Review. - Dual role of pericyte α6β1-integrin in tumour blood vessels.
Reynolds LE, D'Amico G, Lechertier T, Papachristodoulou A, Muñoz-Félix JM, De Arcangelis A, Baker M, Serrels B, Hodivala-Dilke KM. Reynolds LE, et al. J Cell Sci. 2017 May 1;130(9):1583-1595. doi: 10.1242/jcs.197848. Epub 2017 Mar 13. J Cell Sci. 2017. PMID: 28289267 Free PMC article. - Podosomes and invadopodia: tools to breach vascular basement membrane.
Seano G, Primo L. Seano G, et al. Cell Cycle. 2015;14(9):1370-4. doi: 10.1080/15384101.2015.1026523. Cell Cycle. 2015. PMID: 25789660 Free PMC article. Review.
Cited by
- Actin cytoskeleton in angiogenesis.
Yadunandanan Nair N, Samuel V, Ramesh L, Marib A, David DT, Sundararaman A. Yadunandanan Nair N, et al. Biol Open. 2022 Dec 15;11(12):bio058899. doi: 10.1242/bio.058899. Epub 2022 Nov 29. Biol Open. 2022. PMID: 36444960 Free PMC article. Review. - Pivotal role for decorin in angiogenesis.
Järveläinen H, Sainio A, Wight TN. Järveläinen H, et al. Matrix Biol. 2015 Apr;43:15-26. doi: 10.1016/j.matbio.2015.01.023. Epub 2015 Feb 7. Matrix Biol. 2015. PMID: 25661523 Free PMC article. Review. - Spatial models of pattern formation during phagocytosis.
Herron JC, Hu S, Liu B, Watanabe T, Hahn KM, Elston TC. Herron JC, et al. PLoS Comput Biol. 2022 Oct 3;18(10):e1010092. doi: 10.1371/journal.pcbi.1010092. eCollection 2022 Oct. PLoS Comput Biol. 2022. PMID: 36190993 Free PMC article. - A PKA/cdc42 Signaling Axis Restricts Angiogenic Sprouting by Regulating Podosome Rosette Biogenesis and Matrix Remodeling.
MacKeil JL, Brzezinska P, Burke-Kleinman J, Craig AW, Nicol CJB, Maurice DH. MacKeil JL, et al. Sci Rep. 2019 Feb 20;9(1):2385. doi: 10.1038/s41598-018-37805-y. Sci Rep. 2019. PMID: 30787359 Free PMC article. - Research progress on the mechanism of angiogenesis in wound repair and regeneration.
Shi Z, Yao C, Shui Y, Li S, Yan H. Shi Z, et al. Front Physiol. 2023 Nov 27;14:1284981. doi: 10.3389/fphys.2023.1284981. eCollection 2023. Front Physiol. 2023. PMID: 38089479 Free PMC article. Review.
References
- Hallmann R, et al. Expression and function of laminins in the embryonic and mature vasculature. Physiol. Rev. 2005;85:979–1000. - PubMed
- Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat. Med. 2003;9:669–676. - PubMed
- Inoue M, Hager JH, Ferrara N, Gerber HP, Hanahan D. VEGF-A has a critical, nonredundant role in angiogenic switching and pancreatic beta cell carcinogenesis. Cancer Cell. 2002;1:193–202. - PubMed
- Galvez BG, Matias-Roman S, Albar JP, Sanchez-Madrid F, Arroyo AG. Membrane type 1-matrix metalloproteinase is activated during migration of human endothelial cells and modulates endothelial motility and matrix remodeling. J. Biol. Chem. 2001;276:37491–37500. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases