Investigation of 6-[¹⁸F]-fluoromaltose as a novel PET tracer for imaging bacterial infection - PubMed (original) (raw)
Investigation of 6-[¹⁸F]-fluoromaltose as a novel PET tracer for imaging bacterial infection
Gayatri Gowrishankar et al. PLoS One. 2014.
Abstract
Despite advances in the field of nuclear medicine, the imaging of bacterial infections has remained a challenge. The existing reagents suffer from poor sensitivity and specificity. In this study we investigate the potential of a novel PET (positron emission tomography) tracer that overcomes these limitations.
Methods: 6-[¹⁸F]-fluoromaltose was synthesized. Its behavior in vitro was evaluated in bacterial and mammalian cultures. Detailed pharmacokinetic and biodistribution profiles for the tracer were obtained from a murine model.
Results: 6-[¹⁸F]-fluoromaltose is taken up by multiple strains of pathogenic bacteria. It is not taken up by mammalian cancer cell lines. 6-[¹⁸F]-fluoromaltose is retained in infected muscles in a murine model of bacterial myositis. It does not accumulate in inflamed tissue.
Conclusion: We have shown that 6-[¹⁸F]-fluoromaltose can be used to image bacterial infection in vivo with high specificity. We believe that this class of agents will have a significant impact on the clinical management of patients.
Conflict of interest statement
Competing Interests: The authors have a patent on the imaging probe described in the manuscript. Patent title: PROBES AND METHODS OF IMAGING A BACTERIAL INFECTION. Serial No. 14/204,402. Filed: March 11, 2014. Docket No. 221907-1950. One of the authors, Dr. Erwan Jouannot, is an employee of Sanofi Inc. This does not alter the authors' adherence to PLOS ONE editorial policies and criteria. Sanjiv Sam Gambhir is on the editorial board for PLOS ONE. This does not alter the authors' adherence to PLOS ONE editorial policies and criteria.
Figures
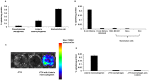
Figure 1. In vitro characterization of 6-[18F]-fluoromaltose.
A) Uptake of 6-[18F]-fluoromaltose in the indicated strains of bacteria for 60 minutes. B) 1 hour uptake of 6-[18F]-fluoromaltose in the mammalian cell lines, MDA MB231 and HeLa and its uptake in E.coli in the presence of 1 mM maltose. C) Bioluminescence imaging of a macrophage cell line J774 infected with a bioluminescent strain of Listeria monocytogenes. D) 1 hour uptake of 6-[18F]-fluoromaltose in the bioluminescent strain of Listeria monocytogenes and in macrophage cell line J774 with and without intracellular Listeria infections.
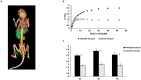
Figure 2. In vivo characterization of 6-[18F]-fluoromaltose.
A) 3D color map from a PET/CT scan of a mouse bearing E.coli induced infection on the left thigh (red arrow) 1 hr after tail-vein injection of 7.4MBq of 6-[18F]-fluoromaltose. B) Region of interest analysis (ROIs) from PET/CT images at the indicated time points (n = 4 for each time point) * indicates statistical significance with p<0.05. C) Time activity curve showing accumulation of 6-[18F]-fluoromaltose in the infected muscle (n = 3).
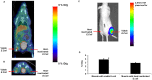
Figure 3. Specificity of 6-[18F]-fluoromaltose for viable bacteria.
A) A coronal slice from a PET/CT image of a mouse bearing 108 CFU of viable bioluminescent E.coli on the right thigh (red arrow) and 108 CFU of heat-inactivated E.coli on the left thigh, 1hr after tail-vein injection of 7.4MBq of 6-[18F]-fluoromaltose B) A transverse slice from the same mouse shown in A), with arrows indicating sites of viable and heat inactivated bacteria. C) Bioluminescent image of the mouse shown in A). D) ROI analysis from PET/CT scan of mice (n = 3). * indicates statistical significance.
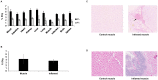
Figure 4. Uptake of 6-[18F]-fluoromaltose in infection versus inflammation.
A) Ex-vivo biodistribution of 6-[18F]-fluoromaltose in mice bearing E.coli induced myositis, 2 h and 4 h after tail-vein injection of 7.4MBq of tracer. B) Ex-vivo biodistribution of 6-[18F]-fluoromaltose in mice bearing turpentine oil induced sterile abscess, 2 h after tail-vein injection of 7.4MBq of tracer. C) Representative gram stained muscle sections with a black arrow indicating presence of E.coli in the infected muscle section. D) Representative H&E stained muscle sections showing neutrophil infiltration in inflamed muscle.
Similar articles
- Synthesis of [¹⁸F]-labelled maltose derivatives as PET tracers for imaging bacterial infection.
Namavari M, Gowrishankar G, Hoehne A, Jouannot E, Gambhir SS. Namavari M, et al. Mol Imaging Biol. 2015 Apr;17(2):168-76. doi: 10.1007/s11307-014-0793-5. Mol Imaging Biol. 2015. PMID: 25277604 Free PMC article. - Synthesis and preclinical evaluation of novel 18F-vancomycin-based tracers for the detection of bacterial infections using positron emission tomography.
Spoelstra GB, Blok SN, Reali Nazario L, Noord L, Fu Y, Simeth NA, IJpma FFA, van Oosten M, van Dijl JM, Feringa BL, Szymanski W, Elsinga PH. Spoelstra GB, et al. Eur J Nucl Med Mol Imaging. 2024 Jul;51(9):2583-2596. doi: 10.1007/s00259-024-06717-7. Epub 2024 Apr 22. Eur J Nucl Med Mol Imaging. 2024. PMID: 38644432 Free PMC article. - Specific Imaging of Bacterial Infection Using 6″-18F-Fluoromaltotriose: A Second-Generation PET Tracer Targeting the Maltodextrin Transporter in Bacteria.
Gowrishankar G, Hardy J, Wardak M, Namavari M, Reeves RE, Neofytou E, Srinivasan A, Wu JC, Contag CH, Gambhir SS. Gowrishankar G, et al. J Nucl Med. 2017 Oct;58(10):1679-1684. doi: 10.2967/jnumed.117.191452. Epub 2017 May 10. J Nucl Med. 2017. PMID: 28490473 Free PMC article. - Biological evaluation of 3-[(18)F]fluoro-α-methyl-D-tyrosine (D-[(18)F]FAMT) as a novel amino acid tracer for positron emission tomography.
Ohshima Y, Hanaoka H, Tominaga H, Kanai Y, Kaira K, Yamaguchi A, Nagamori S, Oriuchi N, Tsushima Y, Endo K, Ishioka NS. Ohshima Y, et al. Ann Nucl Med. 2013 May;27(4):314-24. doi: 10.1007/s12149-013-0687-7. Epub 2013 Jan 23. Ann Nucl Med. 2013. PMID: 23337966 - Biological Evaluation of d-[18F]Fluoroalanine and d-[18F]Fluoroalanine-_d_3 as Positron Emission Tomography Imaging Tracers for Bacterial Infection.
Li K, Tolentino Collado J, Marden JA, Pollard AC, Guo S, Tonge PJ, Qu W. Li K, et al. J Med Chem. 2024 Aug 22;67(16):13975-13984. doi: 10.1021/acs.jmedchem.4c00783. Epub 2024 Jul 31. J Med Chem. 2024. PMID: 39082959
Cited by
- Preclinical studies and prospective clinical applications for bacteria-targeted imaging: the future is bright.
Heuker M, Gomes A, van Dijl JM, van Dam GM, Friedrich AW, Sinha B, van Oosten M. Heuker M, et al. Clin Transl Imaging. 2016;4:253-264. doi: 10.1007/s40336-016-0190-y. Epub 2016 Jul 16. Clin Transl Imaging. 2016. PMID: 27512688 Free PMC article. Review. - PET Radiopharmaceuticals for Specific Bacteria Imaging: A Systematic Review.
Auletta S, Varani M, Horvat R, Galli F, Signore A, Hess S. Auletta S, et al. J Clin Med. 2019 Feb 6;8(2):197. doi: 10.3390/jcm8020197. J Clin Med. 2019. PMID: 30736324 Free PMC article. Review. - The Potential of Metabolic Imaging.
Di Gialleonardo V, Wilson DM, Keshari KR. Di Gialleonardo V, et al. Semin Nucl Med. 2016 Jan;46(1):28-39. doi: 10.1053/j.semnuclmed.2015.09.004. Semin Nucl Med. 2016. PMID: 26687855 Free PMC article. Review. - Bacterial infection imaging with [18F]fluoropropyl-trimethoprim.
Sellmyer MA, Lee I, Hou C, Weng CC, Li S, Lieberman BP, Zeng C, Mankoff DA, Mach RH. Sellmyer MA, et al. Proc Natl Acad Sci U S A. 2017 Aug 1;114(31):8372-8377. doi: 10.1073/pnas.1703109114. Epub 2017 Jul 17. Proc Natl Acad Sci U S A. 2017. PMID: 28716936 Free PMC article. - Maltohexaose-indocyanine green (MH-ICG) for near infrared imaging of endocarditis.
Takemiya K, Røise JJ, He M, Taing C, Rodriguez AG, Murthy N, Goodman MM, Taylor WR. Takemiya K, et al. PLoS One. 2021 Mar 1;16(3):e0247673. doi: 10.1371/journal.pone.0247673. eCollection 2021. PLoS One. 2021. PMID: 33647027 Free PMC article.
References
- Akhtar MS, Qaisar A, Irfanullah J, Iqbal J, Khan B, et al. (2005) Antimicrobial peptide 99mTc-ubiquicidin 29-41 as human infection-imaging agent: clinical trial. J Nucl Med 46: 567–573. - PubMed
- Bunschoten A, Welling MM, Termaat MF, Sathekge M, van Leeuwen FW (2013) Development and prospects of dedicated tracers for the molecular imaging of bacterial infections. Bioconjug Chem 24: 1971–1989. - PubMed
- Machens HG, Pallua N, Becker M, Mailaender P, Schaller E, et al. (1996) Technetium-99m human immunoglobulin (HIG): a new substance for scintigraphic detection of bone and joint infections. Microsurgery 17: 272–277. - PubMed
- Sasser TA, Van Avermaete AE, White A, Chapman S, Johnson JR, et al. (2013) Bacterial infection probes and imaging strategies in clinical nuclear medicine and preclinical molecular imaging. Curr Top Med Chem 13: 479–487. - PubMed
MeSH terms
Substances
Grants and funding
The authors received no specific funding for this work.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical