Breast cancer: coordinated regulation of CCL2 secretion by intracellular glycosaminoglycans and chemokine motifs - PubMed (original) (raw)
Breast cancer: coordinated regulation of CCL2 secretion by intracellular glycosaminoglycans and chemokine motifs
Yaeli Lebel-Haziv et al. Neoplasia. 2014 Sep.
Abstract
The chemokine CCL2 (MCP-1) has been identified as a prominent tumor-promoting factor in breast cancer. The major source for CCL2 is in the tumor cells; thus, identifying the mechanisms regulating CCL2 release by these cells may enable the future design of modalities inhibiting CCL2 secretion and consequently reduce tumorigenicity. Using cells deficient in expression of glycosaminoglycans (GAGs) and short hairpin RNAs reducing heparan sulfate (HS) and chondroitin sulfate (CS) expression, we found that intracellular HS and CS (=GAGs) partly controlled the trafficking of CCL2 from the Golgi toward secretion. Next, we determined the secretion levels of GFP-CCL2-WT and GFP-CCL2-variants mutated in GAG-binding domains and/or in the 40s loop of CCL2 ((45)TIVA(48)). We have identified partial roles for R18+K19, H66, and the (45)TIVA(48) motif in regulating CCL2 secretion. We have also demonstrated that in the absence of R24 or R18+K19+(45)TIVA(48), the secretion of CCL2 by breast tumor cells was almost abolished. Analyses of the intracellular localization of GFP-CCL2-mutants in the Golgi or the endoplasmic reticulum revealed particular intracellular processes in which these CCL2 sequences controlled its intracellular trafficking and secretion. The R24, (45)TIVA(48) and R18+K19+(45)TIVA(48) domains controlled CCL2 secretion also in other cell types. We propose that targeting these chemokine regions may lead to reduced secretion of CCL2 by breast cancer cells (and potentially also by other malignant cells). Such a modality may limit tumor growth and metastasis, presumably without affecting general immune activities (as discussed below).
Copyright © 2014 Neoplasia Press, Inc. Published by Elsevier Inc. All rights reserved.
Figures
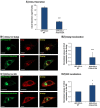
Figure 1
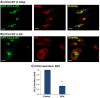
CCL2 WT takes the ER-to-Golgi route toward secretion. MDA-CCL2-low cells were transfected to express GFP-CCL2 WT. Additional characteristics of the transfected cells are shown in Figure W1. (A and B) After 48 hours, the intracellular localization of CCL2 was determined by confocal analyses of the GFP signal. (A) GFP-CCL2 WT expression in the Golgi. In parallel to expression of GFP-CCL2 WT, the expression of the Golgi marker α-mannosidase IB was determined by antibodies. (B) GFP-CCL2 WT expression in the ER. In parallel to expression of GFP-CCL2 WT, the expression of the ER marker calnexin was determined by antibodies. In A and B, negative controls included staining by non-relevant isotype-matched antibodies (data not shown). The results are representatives of many pictures that were taken in n > 3 independent experiments. Bar, 10 μm. (C) The secretion of CCL2 required for ER-to-Golgi transport of the chemokine. The cells were treated by BFA (5-10 μg/ml; 2 hours), and the levels of CCL2 in cell supernatants were determined by sandwich ELISAs, at the linear range of absorbance. Untreated cells (control) were given the value of 1. C presents normalized results of a representative experiment of n > 3 independent experiments, where **P < .01 comparing between BFA-treated and untreated cells. The P value was calculated from the original test values, before normalization. Inhibition levels by BFA were 70.9 ± 15.2% in the different experiments.
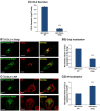
Figure 2
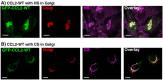
CCL2 WT is localized with HS and CS in the Golgi. MDA-CCL2-low cells were transfected to express GFP-CCL2 WT. After 48 hours, the intracellular localization of the chemokine was determined by confocal analyses of the GFP signal. (A) Co-localization of GFP-CCL2 WT with HS in the Golgi. The expression patterns of HS and of the Golgi marker α-mannosidase IB were determined by antibodies. (B) Co-localization of GFP-CCL2 WT with CS in the Golgi. The expression patterns of CS and of the Golgi marker α-mannosidase IB were determined by antibodies. In both parts of the figure, negative controls included staining by non-relevant isotype-matched antibodies (data not shown). The results are representatives of many pictures that were taken in n > 3 independent experiments. Bar, 10 μm.
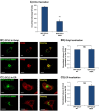
Figure 3
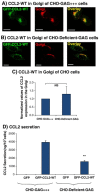
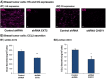
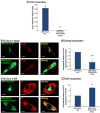
Intracellular GAGs are partly required for CCL2 secretion and for the transport of CCL2 WT from the Golgi toward secretion. CHO-GAG+++ cells and CHO-deficient GAG cells were transfected to express GFP-CCL2 WT. Additional characteristics of the two CHO cell types are shown in Figure W2. (A and B) Localization of GFP-CCL2 WT in the Golgi of CHO-GAG+++ and of CHO-deficient GAG cells, determined by confocal analyses of the GFP signal. In parallel to expression of GFP-CCL2 WT, the expression of the Golgi marker α-mannosidase IB was determined by antibodies. (A) The localization of GFP-CCL2 WT in the Golgi of CHO-GAG+++ cells. (B) The localization of GFP-CCL2 WT in the Golgi of CHO-deficient GAG cells. In A and B, the negative controls included staining by non-relevant isotype-matched antibodies (data not shown). The results are representatives of many pictures that were taken in n > 3 independent experiments. Bar, 10 μm. (C) Quantitative analyses of the co-localization obtained between GFP-CCL2 WT and the Golgi marker α-mannosidase IB in CHO-GAG+++ and in CHO-deficient GAG cells, performed by the Slidebook program. In C, the results are based on analyses of many pictures that were taken in n > 3 independent experiments and are presented as mean ± SD, where the values of CHO-GAG+++ cells were given the value of 1. NS, not significant. (D) The secretion of GFP-CCL2 WT by CHO-GAG+++ cells and CHO-deficient GAG cells. The levels of CCL2 in cell supernatants were determined by sandwich ELISAs, at the linear range of absorbance. **P < .01 comparing between CCL2 secretion levels in CHO-GAG+++ cells _versus_ CHO-deficient GAG cells, with inhibition levels of 59.4 ± 16.2% in the different experiments. In D, the results are representatives of _n_ > 3 independent experiments.
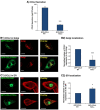
Figure 4
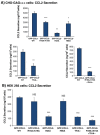
HS and CS partly mediate the secretion of CCL2 by breast tumor cells. MDA-CCL2-high cells were infected by shRNAs directed against the enzymes that synthesize HS or CS or by control non-relevant shRNAs. After 48 hours, the expression of HS or CS was determined, and in parallel, the secretion of endogenous CCL2 was evaluated. (A) Down-regulation of HS expression (A1) or CS expression (A2) after infection by shRNA for EXT2 (A1) or for CHSY1 (A2). The shRNAs were selected out of several shRNAs that were tested for their ability to downregulate HS or CS expression. HS or CS expression was determined by antibodies using confocal analyses. Negative controls included cells transfected by non-relevant shRNAs and staining by non-relevant isotype-matched antibodies (data not shown). In A, the results are representatives of many pictures that were taken in n > 3 independent experiments. Bar, 10 μm. (B) The levels of CCL2 in cell supernatants were determined by sandwich ELISA in the linear range of absorbance, in cells expressing shRNAs for EXT2 (B1) or shRNA for CHSY1 (B2). *P < .05 and **_P_ < .01 comparing cells transfected with control shRNA and shRNAs for EXT2 or CHSY1. The inhibition levels in cells expressing shRNA downregulating EXT2 were 37.6 ± 6.2%, and for shRNA downregulating CHSY1, they were 36.1 ± 3.8%. In B, the results are representatives of _n_ > 3 independent experiments.
Figure 5
The secretion of GFP-CCL2-R18A+K19A by breast tumor cells is partly inhibited, and the mutated chemokine shows reduced propensity for Golgi localization. MDA-CCL2-low cells were transfected to express GFP-CCL2 WT or GFP-CCL2-R18A+K19A. Similar expression levels of GFP-CCL2-R18A+K19A, compared to GFP-CCL2 WT, were validated by FACS analyses (Figure W5_A_). (A) The levels of CCL2 in cell supernatants were determined 48 hours following transfection by sandwich ELISAs, at the linear range of absorbance. In A, the results are representatives of n > 3 independent experiments. (B) The expression of GFP-CCL2-R18A+K19A versus GFP-CCL2 WT in the Golgi was determined on the basis of the GFP signal, combined with antibodies to the Golgi marker α-mannosidase IB, using confocal analyses. Negative controls included staining by non-relevant isotype-matched antibodies (data not shown). Determination of localization was performed qualitatively (B1) and quantitatively by the Slidebook program (B2). Bar, 10 μm. (C) The expression of GFP-CCL2-R18A+K19A versus GFP-CCL2 WT in the ER was determined on the basis of the GFP signal combined with antibodies to the ER marker calnexin, using confocal analyses. Negative controls included staining by non-relevant isotype-matched antibodies (data not shown). Determination of localization was performed qualitatively (C1) and quantitatively by the ImageJ program (C2). Bar, 10 μm. In B1 and C1, the results are representatives of many pictures that were taken in n > 3 independent experiments. In B2 and C2, the results are mean ± SD values obtained from many pictures that were analyzed, where the results of GFP-CCL2 WT−expressing cells were given the value of 1. Throughout the figure, *P < .05 and ***P < .001 comparing GFP-CCL2 WT and GFP-CCL2-R18A+K19A. Table 2 provides a summary of all the results presented in the figure.
Figure 6
The secretion of GFP-CCL2-R24A by breast tumor cells is almost completely inhibited, and the mutated chemokine shows reduced propensity for Golgi localization. MDA-CCL2-low cells were transfected to express GFP-CCL2 WT or GFP-CCL2-R24A. Similar expression levels of GFP-CCL2-R24A compared to GFP-CCL2 WT were validated by FACS analyses (Figure W5_B_). All additional details (including number of repeats and modes of data presentation) are similar to those of Figure 5, albeit with the GFP-CCL2-R24A mutant. Throughout the figure, ***P < .001 comparing GFP-CCL2 WT and GFP-CCL2-R24A. Bar, 10 μm. Table 2 provides a summary of all the results presented in the figure.
Figure 7
The secretion of GFP-CCL2-H66A by breast tumor cells is partly inhibited, and the mutated chemokine shows normal Golgi and ER localization. MDA-CCL2-low cells were transfected to express GFP-CCL2 WT or GFP-CCL2-H66A. Similar expression levels of GFP-CCL2-H66A compared to GFP-CCL2 WT were validated by FACS analyses (Figure W5_C_). All additional details (including number of repeats and modes of data presentation) are similar to those of Figure 5, albeit with the GFP-CCL2-H66A mutant. Throughout the figure, **P < .01 comparing GFP-CCL2 WT and GFP-CCL2-H66A. NS, not significant. Bar, 10 μm. Table 2 provides a summary of all the results presented in the figure.
Figure 8
The secretion of GFP-CCL2-TIVA−− by breast tumor cells is partly inhibited, and the mutated chemokine shows reduced propensity for Golgi localization. MDA-CCL2-low cells were transfected to express GFP-CCL2 WT or GFP-CCL2-TIVA−−. Similar expression levels of GFP-CCL2-TIVA−− compared to GFP-CCL2 WT were validated by FACS analyses (Figure W5_D_). All additional details (including number of repeats and modes of data presentation) are similar to those of Figure 5, albeit with the GFP-CCL2-TIVA−− mutant. Throughout the figure, *P < .05 and ***P < .001 comparing GFP-CCL2 WT and GFP-CCL2-TIVA−−. Bar, 10 μm. Table 2 provides a summary of all the results presented in the figure.
Figure 9
The secretion of GFP-CCL2-R18A+K19A+TIVA−− by breast tumor cells is completely inhibited, and the mutated chemokine shows reduced propensity for Golgi localization. MDA-CCL2-low cells were transfected to express GFP-CCL2 WT or GFP-CCL2-R18A+K19A+TIVA−−. Similar expression levels of GFP-CCL2-R18A+K19A+TIVA−− compared to GFP-CCL2 WT were validated by FACS analyses (Figure W5_E_). All additional details (including number of repeats and modes of data presentation) are similar to those of Figure 5, albeit with the GFP-CCL2-R18A+K19A+TIVA−− mutant. Throughout the figure, **P < .01 and ***P < .001 comparing GFP-CCL2 WT and GFP-CCL2-R18A+K19A+TIVA−−. Bar, 10 μm. Table 2 provides a summary of all the results presented in the figure.
Figure 10
The general use of CCL2 GAG-binding domains and its 40s loop by different cell types. CHO-GAG+++ cells (A) and HEK 293 cells (B) were transfected to express GFP-CCL2 WT or GFP-CCL2 mutants: GFP-CCL2-R18A+K19A, GFP-CCL2-R24A, GFP-CCL2-H66A, GFP-CCL2-TIVA−−, and GFP-CCL2-R18A+K19A+TIVA−−. Similar expression levels of the WT and mutated variants of CCL2 were validated by FACS analyses (Figure W6 for CHO-GAG+++ cells and Figure W7 for HEK 293 cells). The levels of CCL2 in cell supernatants were determined 48 hours following transfection by sandwich ELISAs, at the linear range of absorbance. *P < .05, **P < .01 and ***P < .001 for GFP-CCL2 mutants compared to GFP-CCL2 WT. NS, not significant. The results are representatives of n ≥ 3 independent experiments. Table 3 provides a summary of all the results.
Similar articles
- Mechanisms regulating the secretion of the promalignancy chemokine CCL5 by breast tumor cells: CCL5's 40s loop and intracellular glycosaminoglycans.
Soria G, Lebel-Haziv Y, Ehrlich M, Meshel T, Suez A, Avezov E, Rozenberg P, Ben-Baruch A. Soria G, et al. Neoplasia. 2012 Jan;14(1):1-19. doi: 10.1593/neo.111122. Neoplasia. 2012. PMID: 22355269 Free PMC article. - Designing a mutant CCL2-HSA chimera with high glycosaminoglycan-binding affinity and selectivity.
Gerlza T, Winkler S, Atlic A, Zankl C, Konya V, Kitic N, Strutzmann E, Knebl K, Adage T, Heinemann A, Weis R, Kungl AJ. Gerlza T, et al. Protein Eng Des Sel. 2015 Aug;28(8):231-40. doi: 10.1093/protein/gzv025. Epub 2015 May 11. Protein Eng Des Sel. 2015. PMID: 25969511 - Multiple glycosaminoglycan-binding epitopes of monocyte chemoattractant protein-3/CCL7 enable it to function as a non-oligomerizing chemokine.
Salanga CL, Dyer DP, Kiselar JG, Gupta S, Chance MR, Handel TM. Salanga CL, et al. J Biol Chem. 2014 May 23;289(21):14896-912. doi: 10.1074/jbc.M114.547737. Epub 2014 Apr 11. J Biol Chem. 2014. PMID: 24727473 Free PMC article. - Sulfation of Glycosaminoglycans and Its Implications in Human Health and Disorders.
Soares da Costa D, Reis RL, Pashkuleva I. Soares da Costa D, et al. Annu Rev Biomed Eng. 2017 Jun 21;19:1-26. doi: 10.1146/annurev-bioeng-071516-044610. Epub 2017 Feb 2. Annu Rev Biomed Eng. 2017. PMID: 28226217 Review. - The inflammatory chemokines CCL2 and CCL5 in breast cancer.
Soria G, Ben-Baruch A. Soria G, et al. Cancer Lett. 2008 Aug 28;267(2):271-85. doi: 10.1016/j.canlet.2008.03.018. Epub 2008 Apr 24. Cancer Lett. 2008. PMID: 18439751 Review.
Cited by
- Exploring the multifaceted role of obesity in breast cancer progression.
Kakkat S, Suman P, Turbat-Herrera EA, Singh S, Chakroborty D, Sarkar C. Kakkat S, et al. Front Cell Dev Biol. 2024 Jul 8;12:1408844. doi: 10.3389/fcell.2024.1408844. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 39040042 Free PMC article. Review. - The Importance of Breast Adipose Tissue in Breast Cancer.
Kothari C, Diorio C, Durocher F. Kothari C, et al. Int J Mol Sci. 2020 Aug 11;21(16):5760. doi: 10.3390/ijms21165760. Int J Mol Sci. 2020. PMID: 32796696 Free PMC article. Review. - MicroRNA-155 governs SHIP-1 expression and localization in NK cells and regulates subsequent infiltration into murine AT3 mammary carcinoma.
Kandell WM, Donatelli SS, Trinh TL, Calescibetta AR, So T, Tu N, Gilvary DL, Chen X, Cheng P, Adams WA, Chen YK, Liu J, Djeu JY, Wei S, Eksioglu EA. Kandell WM, et al. PLoS One. 2020 Feb 10;15(2):e0225820. doi: 10.1371/journal.pone.0225820. eCollection 2020. PLoS One. 2020. PMID: 32040476 Free PMC article. - The dual role of the glycosaminoglycan chondroitin-6-sulfate in the development, progression and metastasis of cancer.
Pudełko A, Wisowski G, Olczyk K, Koźma EM. Pudełko A, et al. FEBS J. 2019 May;286(10):1815-1837. doi: 10.1111/febs.14748. Epub 2019 Feb 5. FEBS J. 2019. PMID: 30637950 Free PMC article. Review. - Visfatin Mediates SCLC Cells Migration across Brain Endothelial Cells through Upregulation of CCL2.
Liu T, Miao Z, Jiang J, Yuan S, Fang W, Li B, Chen Y. Liu T, et al. Int J Mol Sci. 2015 May 18;16(5):11439-51. doi: 10.3390/ijms160511439. Int J Mol Sci. 2015. PMID: 25993304 Free PMC article.
References
- Colotta F., Allavena P., Sica A., Garlanda C., Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis. 2009;30:1073–1081. - PubMed
- Hanahan D., Coussens L.M. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012;21:309–322. - PubMed
- Balkwill F.R., Mantovani A. Cancer-related inflammation: common themes and therapeutic opportunities. Semin Cancer Biol. 2012;22:33–40. - PubMed
- Borsig L., Wolf M.J., Roblek M., Lorentzen A., Heikenwalder M. Inflammatory chemokines and metastasis—tracing the accessory. Oncogene. 2013;33:3217–3224. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous