Alpha-synuclein as a pathological link between chronic traumatic brain injury and Parkinson's disease - PubMed (original) (raw)
Alpha-synuclein as a pathological link between chronic traumatic brain injury and Parkinson's disease
Sandra A Acosta et al. J Cell Physiol. 2015 May.
Abstract
The long-term consequences of traumatic brain injury (TBI) are closely associated with the development of histopathological deficits. Notably, TBI may predispose long-term survivors to age-related neurodegenerative diseases, such as Parkinson's disease (PD), which is characterized by a gradual degeneration of the nigrostriatal dopaminergic neurons. However, preclinical studies on the pathophysiological changes in substantia nigra (SN) after chronic TBI are lacking. In the present in vivo study, we examined the pathological link between PD-associated dopaminergic neuronal loss and chronic TBI. Sixty days post-TBI, rats were euthanized and brain tissues harvested. Immunostaining was performed using tyrosine hydroxylase (TH), an enzyme required for the synthesis of dopamine in neurons, α-synuclein, a presynaptic protein that plays a role in synaptic vesicle recycling, and major histocompatibility complex II (MHCII), a protein found in antigen presenting cells such as inflammatory microglia cells, all key players in PD pathology. Unbiased stereology analyses revealed significant decrease of TH-positive expression in the surviving dopaminergic neurons of the SN pars compacta (SNpc) relative to sham control. In parallel, increased α-synuclein accumulation was detected in the ipsilateral SN compared to the contralateral SN in TBI animals or sham control. In addition, exacerbation of MHCII+ cells was recognized in the SN and cerebral peduncle ipsilateral to injury relative to contralateral side and sham control. These results suggest α-synuclein as a pathological link between chronic effects of TBI and PD symptoms as evidenced by significant overexpression and abnormal accumulation of α-synuclein in inflammation-infiltrated SN of rats exposed to chronic TBI.
© 2014 The Authors. Journal of Cellular Physiology Published by Wiley Periodicals, Inc.
Figures
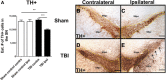
Figure 1
Downregulation of TH-positive dopaminergic neurons in the SNpc in chronic TBI. Arrows indicate downregulation of TH-positive dopaminergic neurons in TBI ipsilateral SNpc. Quantification of TH immunostaining reflects mean estimated number of TH-positive dopaminergic cells in the SNpc (A). SNpc, F3,20 = 11.79; P < 0.0001. Photomicrographs correspond to representative SN in coronal sections immunostained with TH antibody. (B) Sham contralateral SNpc, (C) Sham ipsilateral SNpc, (D) TBI contralateral SNpc, (E) TBI ipsilateral SNpc. Scale bar = 100 μm. Significance at P's < 0.05.
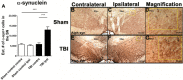
Figure 2
Overexpression of α-synuclein-positive cells in the SNpc in chronic TBI rats. Upregulation of α-synuclein-positive cells in the ipsilateral side of SNpc of chronic TBI rats relative to contralateral side and sham control. Arrows indicate positive expression of α-synuclein-positive cells in TBI ipsilateral SNpc. Quantification of α-synuclein immunostaining reflects mean estimated number of α-synuclein-positive cells in the SNpc (A). SNpc, F3,20 = 17.32; P < 0.0001. Photomicrographs correspond to representative SN in coronal sections immunostained with α-synuclein antibody. (B) Sham contralateral SNpc, (C) Sham ipsilateral SNpc, (D) magnification of sham ipsilateral SNpc, (E) TBI contralateral SNpc, (F) TBI ipsilateral SNpc, (G) magnification of TBI ipsilateral SNpc. Arrows indicate expression of α-synuclein in cells in all groups within the SNpc (Figs. 2B–E). Scale bar for B, C, E, F = 500 μm and D, G =0.1 μm. Significance at P's < 0.05.
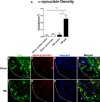
Figure 3
Quantification of α-synuclein density in dopaminergic neurons in the SNpc of chronic TBI rat. Results revealed upregulation of α-synuclein as measured by α-synuclein density in TH-positive dopaminergic neurons in the ipsilateral side of SNpc of chronic TBI rats relative to contralateral side and sham control. (A) Arrows denote positive expression of α-synuclein detected in the cytoplasm of the soma and neurites. Quantification of total α-synuclein immunostaining reflects the density of α-synuclein-positive expression in the cytoplasm and neurites in the SNpc (A). F3,20 = 15.37; P < 0.0001. Confocal photomicrographs of positive expression of TH (green) (B–F), α-synuclein (red) (C–G), and Hoechst (blue) (D–H) within the SNpc dopaminergic neurons of sham control rats and TBI rats at 60 days post-TBI. (E) Colocalization of TH, α-synuclein, and Hoechst shows minimum expression of α-synuclein in dopaminergic neurons of the SNpc contralateral side and sham control rats. (I) Colocalization of TH, α-synuclein and Hoechst shows positive expression of α-synuclein in the soma of dopaminergic neurons (yellow arrows) and along dendrites and axonal projections (white arrows) of the ipsilateral SNpc of 60 days post-TBI. Scale bar: 50 μm. Significance at P's < 0.05.
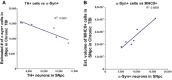
Figure 4
Correlation of TH-positive expression vs. α-synuclein overexpression and MHCII+ cells vs. α-synuclein. Mean estimated number of α-synuclein expressing neurons negatively correlates (A) with the number of TH-positive neurons in the SNpc ipsilateral to TBI (Pearson r = −0.9115, R2 = −0.8381, P < 0.01). Mean estimated number of α-synuclein expressing neurons positively correlates (B) with the volume of MHCII+ cells in the SNpc ipsilateral to TBI (Pearson r = 0.9412, R2 = 0.8858, P < 0.01). Significance at P's < 0.05.
Figure 5
Upregulation of MHCII+ microgia cells in the SN and CP in chronic TBI rats. Quantification of MHCII immunostaining reflects mean estimated volume of MHCII+ activated microglia cells in the SN and CP in the contralateral and ipsilateral side (A, B). SN, F3,2 = 7.951; P < 0.001; CP, F3,2 = 16.18; P < 0.0001. Arrows indicate MHCII+ activated microglia cells ipsilateral SN and CP from TBI injury. Photomicrographs correspond to representative SN in coronal sections immunostained with MHCII/OX6 antibody. (C) Top part left: SN, sham control (contralateral, ipsilateral, and magnification); top part right: SN, TBI (contralateral, ipsilateral, and magnification); bottom part left: CP, sham control (contralateral, ipsilateral, and magnification); bottom part right: CP, TBI (contralateral, ipsilateral, and magnification); scale bar = 100 μm, magnification = 1 μm. Significance at P's < 0.05.
Figure 6
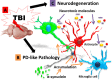
Schematic rendition of the pathological link between TBI and PD via α-synuclein. Overexpression of α-synuclein serves as a pathological link between TBI and PD-associated pathology. After TBI exposure (A), aberrant accumulation of α-synuclein is detected in neurons, microglial cells, and astrocytes (B), leading to propagation of the neurodegeneration (C). The resulting cascade of cell death events contributes to the progressive neurodegeneration associated with increased risk to develop synucleinopathies, such as Parkinson's disease.
Similar articles
- T cell infiltration and upregulation of MHCII in microglia leads to accelerated neuronal loss in an α-synuclein rat model of Parkinson's disease.
Subbarayan MS, Hudson C, Moss LD, Nash KR, Bickford PC. Subbarayan MS, et al. J Neuroinflammation. 2020 Aug 15;17(1):242. doi: 10.1186/s12974-020-01911-4. J Neuroinflammation. 2020. PMID: 32799878 Free PMC article. - Traumatic brain injury in adult rats causes progressive nigrostriatal dopaminergic cell loss and enhanced vulnerability to the pesticide paraquat.
Hutson CB, Lazo CR, Mortazavi F, Giza CC, Hovda D, Chesselet MF. Hutson CB, et al. J Neurotrauma. 2011 Sep;28(9):1783-801. doi: 10.1089/neu.2010.1723. J Neurotrauma. 2011. PMID: 21644813 Free PMC article. - Pedunculopontine cell loss and protein aggregation direct microglia activation in parkinsonian rats.
Elson JL, Yates A, Pienaar IS. Elson JL, et al. Brain Struct Funct. 2016 May;221(4):2319-41. doi: 10.1007/s00429-015-1045-4. Epub 2015 May 20. Brain Struct Funct. 2016. PMID: 25989851 - Pathophysiology and Neuroimmune Interactions Underlying Parkinson's Disease and Traumatic Brain Injury.
Lillian A, Zuo W, Laham L, Hilfiker S, Ye JH. Lillian A, et al. Int J Mol Sci. 2023 Apr 13;24(8):7186. doi: 10.3390/ijms24087186. Int J Mol Sci. 2023. PMID: 37108349 Free PMC article. Review. - Biological links between traumatic brain injury and Parkinson's disease.
Delic V, Beck KD, Pang KCH, Citron BA. Delic V, et al. Acta Neuropathol Commun. 2020 Apr 7;8(1):45. doi: 10.1186/s40478-020-00924-7. Acta Neuropathol Commun. 2020. PMID: 32264976 Free PMC article. Review.
Cited by
- Inflammatory gut as a pathologic and therapeutic target in Parkinson's disease.
Lee JY, Wang ZJ, Moscatello A, Kingsbury C, Cozene B, Farooq J, Saft M, Sadanandan N, Gonzales-Portillo B, Zhang H, Salazar FE, Toledo ARL, Monroy GR, Berlet R, Sanberg CD, Sanberg PR, Borlongan CV. Lee JY, et al. Cell Death Discov. 2022 Sep 24;8(1):396. doi: 10.1038/s41420-022-01175-2. Cell Death Discov. 2022. PMID: 36153318 Free PMC article. - The central role of peripheral inflammation in ischemic stroke.
Monsour M, Borlongan CV. Monsour M, et al. J Cereb Blood Flow Metab. 2023 May;43(5):622-641. doi: 10.1177/0271678X221149509. Epub 2023 Jan 5. J Cereb Blood Flow Metab. 2023. PMID: 36601776 Free PMC article. Review. - Colonic inflammation affects myenteric alpha-synuclein in nonhuman primates.
Resnikoff H, Metzger JM, Lopez M, Bondarenko V, Mejia A, Simmons HA, Emborg ME. Resnikoff H, et al. J Inflamm Res. 2019 May 7;12:113-126. doi: 10.2147/JIR.S196552. eCollection 2019. J Inflamm Res. 2019. PMID: 31123415 Free PMC article. - Quantitative proteomics in A30P*A53T α-synuclein transgenic mice reveals upregulation of Sel1l.
Yan J, Zhang P, Jiao F, Wang Q, He F, Zhang Q, Zhang Z, Lv Z, Peng X, Cai H, Tian B. Yan J, et al. PLoS One. 2017 Aug 3;12(8):e0182092. doi: 10.1371/journal.pone.0182092. eCollection 2017. PLoS One. 2017. PMID: 28771510 Free PMC article. - Pomalidomide Improves Motor Behavioral Deficits and Protects Cerebral Cortex and Striatum Against Neurodegeneration Through a Reduction of Oxidative/Nitrosative Damages and Neuroinflammation After Traumatic Brain Injury.
Huang YN, Greig NH, Huang PS, Chiang YH, Hoffer A, Yang CH, Tweedie D, Chen Y, Ou JC, Wang JY. Huang YN, et al. Cell Transplant. 2024 Jan-Dec;33:9636897241237049. doi: 10.1177/09636897241237049. Cell Transplant. 2024. PMID: 38483119 Free PMC article.
References
- Abdul-Muneer PM, Schuetz H, Wang F, Skotak M, Jones J, Gorantla S, Zimmerman MC, Chandra N, Haorah J. Induction of oxidative and nitrosative damage leads to cerebrovascular inflammation in an animal model of mild traumatic brain injury induced by primary blast. Free Radic Biol Med. 2013;60:282–291. - PMC - PubMed
- Abeliovich A, Schmitz Y, Farinas I, Choi-Lundberg D, Ho WH, Castillo PE, Shinsky N, Verdugo JM, Armanini M, Ryan A, Hynes M, Phillips H, Sulzer D, Rosenthal A. Mice lacking alpha-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron. 2000;25:239–252. - PubMed
- Acosta SA, Tajiri N, Shinozuka K, Ishikawa H, Grimmig B, Diamond DM, Sanberg PR, Bickford PC, Kaneko Y, Borlongan CV. Long-term upregulation of inflammation and suppression of cell proliferation in the brain of adult rats exposed to traumatic brain injury using the controlled cortical impact model. PloS One. 2013;8:e53376. - PMC - PubMed
- Amstrong RA. Quantifying the pathology of neurodegerative disorders: Quantitative measurements, sampling strategies and data analysis. Histopathology. 2003;42:521–529. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous