PCSK9 inhibition fails to alter hepatic LDLR, circulating cholesterol, and atherosclerosis in the absence of ApoE - PubMed (original) (raw)
. 2014 Nov;55(11):2370-9.
doi: 10.1194/jlr.M053207. Epub 2014 Sep 25.
José W A van der Hoorn 2, Joyce Chan 1, Edward Lee 1, Elsbet J Pieterman 2, Kathy Khanh Nguyen 1, Mei Di 1, Susan Shetterly 1, Jie Tang 3, Wen-Chen Yeh 1, Margrit Schwarz 1, J Wouter Jukema 4, Rob Scott 5, Scott M Wasserman 5, Hans M G Princen 2, Simon Jackson 1
Affiliations
- PMID: 25258384
- PMCID: PMC4617138
- DOI: 10.1194/jlr.M053207
PCSK9 inhibition fails to alter hepatic LDLR, circulating cholesterol, and atherosclerosis in the absence of ApoE
Brandon Ason et al. J Lipid Res. 2014 Nov.
Abstract
LDL cholesterol (LDL-C) contributes to coronary heart disease. Proprotein convertase subtilisin/kexin type 9 (PCSK9) increases LDL-C by inhibiting LDL-C clearance. The therapeutic potential for PCSK9 inhibitors is highlighted by the fact that PCSK9 loss-of-function carriers exhibit 15-30% lower circulating LDL-C and a disproportionately lower risk (47-88%) of experiencing a cardiovascular event. Here, we utilized pcsk9(-/-) mice and an anti-PCSK9 antibody to study the role of the LDL receptor (LDLR) and ApoE in PCSK9-mediated regulation of plasma cholesterol and atherosclerotic lesion development. We found that circulating cholesterol and atherosclerotic lesions were minimally modified in pcsk9(-/-) mice on either an LDLR- or ApoE-deficient background. Acute administration of an anti-PCSK9 antibody did not reduce circulating cholesterol in an ApoE-deficient background, but did reduce circulating cholesterol (-45%) and TGs (-36%) in APOE*3Leiden.cholesteryl ester transfer protein (CETP) mice, which contain mouse ApoE, human mutant APOE3*Leiden, and a functional LDLR. Chronic anti-PCSK9 antibody treatment in APOE*3Leiden.CETP mice resulted in a significant reduction in atherosclerotic lesion area (-91%) and reduced lesion complexity. Taken together, these results indicate that both LDLR and ApoE are required for PCSK9 inhibitor-mediated reductions in atherosclerosis, as both are needed to increase hepatic LDLR expression.
Keywords: anti-proprotein convertase subtilisin/kexin type 9 antibody; apolipoprotein E; low density lipoprotein receptor; proprotein convertase subtilisin/kexin type 9.
Copyright © 2014 by the American Society for Biochemistry and Molecular Biology, Inc.
Figures
Fig. 1.
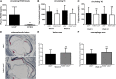
Minimal effect of deleting PCSK9 on circulating lipids or atherosclerosis in LDLR-deficient mice. A: Plasma PCSK9 levels are higher in _ldlr_−/− mice compared with C57Bl/6 mice, consistent with the LDLR being a key clearance mechanism for PCSK9 (n = 9, _ldlr_−/−; n = 12, C57Bl/6). The _pcsk9_−/−/_ldlr_−/− mice exhibit a slight decrease in TC (B) but not TGs (C) relative to _ldlr_−/− mice when fed a WTD (n = 41, _ldlr_−/−; n = 43, _pcsk9_−/−/_ldlr_−/−). D: The aortic sinus was sectioned and stained with VVG to measure lesion area (blue) and Mac-2 to monitor macrophage content (red). D–F: No difference in atherosclerosis development or macrophage accumulation was observed in the aortic sinus for _ldlr_−/− mice relative to _pcsk9_−/−/_ldlr_−/− mice (n = 25 per group). Data represented as the means (bars) ± SD (*P < 0.05, ***P < 0.001, as compared with _ldlr_−/−, two-tailed _t_-test, unpaired).
Fig. 2.
No effect of deleting PCSK9 on circulating lipids or atherosclerosis in APOE-deficient mice. A: Comparable levels of PCSK9 are observed in _apoe_−/− mice compared with C57Bl/6 mice (n = 12, _apoe_−/−; n = 12, C57Bl/6). No significant reduction in circulating TC (B) and TG (C) levels was observed in _pcsk9_−/−/_apoe_−/− mice relative to _apoe_−/− mice (n = 9 (8 weeks), n = 21 (24 weeks) for _pcsk9_−/−/_apoe_−/−; n = 11 (8 weeks), n = 15 (24 weeks) for _apoe_−/−). D–F: The aortic sinus was sectioned and stained with VVG to measure lesion area (blue) and Mac-2 to monitor macrophage content (red), and consistent with these observations, no difference in atherosclerosis development or macrophage accumulation was observed in the aortic sinus for apoe−/− mice relative to _pcsk9_−/−/apoe−/− mice (n = 18, _pcsk9_−/−/_apoe_−/−; n = 20, _apoe_−/−). Data represented as the means (bars) ± SD, two-tailed _t_-test, unpaired, as compared with _apoe_−/−.
Fig. 3.
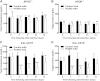
Anti-PCSK9 antibody treatment reduces TC and TG levels in APOE*3Leiden.CETP mice but not _apoe_−/− mice. No significant reduction in TC (A) and only a slight but significant reduction in TGs 5 days posttreatment (B) are observed for anti-PCSK9 antibody-treated _apoe_−/− mice relative to control antibody-treated _apoe_−/− mice [10 mg/kg (sc) day 0, n = 5 per group]. This contrasts results with APOE*3Leiden.CETP mice, where anti-PCSK9 antibody treatment resulted in a significant decrease in TC (C) and TGs (D) (10 mg/kg (sc) day 0, n = 8 per group). Data represented as the means (bars) ± SD, *P < 0.05, **P < 0.01, ****P < 0.0001, as compared with control, two-way ANOVA, Sidak posttest.
Fig. 4.
Anti-PCSK9 antibody treatment reduces atherosclerosis in APOE*3Leiden.CETP mice. Plasma PCSK9 levels (A) in APOE*3Leiden.CETP mice were determined on chow diet and WTD, as well as two weeks after a single injection with anti-PSCK9 antibody (10 mg/kg, sc) in mice fed WTD. Data represented as the means (bars) ± SD (n = 8 per group). *P < 0.01 versus chow; #P < 0.05 versus WD, one-way ANOVA, Tukey posttest. To assess the effect on atherosclerosis, control or anti-PCSK9 antibody was injected sc every 10 days for 14 weeks in APOE*3Leiden.CETP mice. Plasma TC (B) and TGs (C) were measured at 3 and 10 days postinjection in the first and twelfth week of treatment. Data represented as means (bars) ± SD (n = 15 per group). ***P < 0.001 versus control antibody. D: Fast protein liquid chromatography fractionation of pooled plasma samples are shown from week 8. Atherosclerosis development was determined in the aortic sinus of APOE*3Leiden.CETP mice. E–F: Representative pictures of control antibody- and anti-PCSK9 antibody-treated mice are shown. The total lesion area per cross-section (G) was measured and lesion severity (H) was determined. Data represented as means (bars) ± SD (n = 15 per group). ***P < 0.001 versus control antibody.
References
- Steinberg D. 2004. The pathogenesis of atherosclerosis. An interpretive history of the cholesterol controversy: part I. J. Lipid Res. 45: 1583–1593. - PubMed
- Steinberg D. 2005. The pathogenesis of atherosclerosis. An interpretive history of the cholesterol controversy: part II: the early evidence linking hypercholesterolemia to coronary disease in humans. J. Lipid Res. 46: 179–190. - PubMed
- Maxfield F. R., Tabas I. 2005. Role of cholesterol and lipid organization in disease. Nature. 438: 612–621. - PubMed
- Gylling H. 2004. Cholesterol metabolism and its implications for therapeutic interventions in patients with hypercholesterolaemia. Int. J. Clin. Pract. 58: 859–866. - PubMed
- Top B., Koeleman B. P., Gevers Leuven J. A., Havekes L. M., Frants R. R. 1990. Rearrangements in the LDL receptor gene in Dutch familial hypercholesterolemic patients and the presence of a common 4 kb deletion. Atherosclerosis. 83: 127–136. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous