The Sestrins interact with GATOR2 to negatively regulate the amino-acid-sensing pathway upstream of mTORC1 - PubMed (original) (raw)
The Sestrins interact with GATOR2 to negatively regulate the amino-acid-sensing pathway upstream of mTORC1
Lynne Chantranupong et al. Cell Rep. 2014.
Abstract
The mechanistic target of rapamycin complex 1 (mTORC1) kinase is a major regulator of cell growth that responds to numerous environmental cues. A key input is amino acids, which act through the heterodimeric Rag GTPases (RagA or RagB bound to RagC or RagD) in order to promote the translocation of mTORC1 to the lysosomal surface, its site of activation. GATOR2 is a complex of unknown function that positively regulates mTORC1 signaling by acting upstream of or in parallel to GATOR1, which is a GTPase-activating protein (GAP) for RagA or RagB and an inhibitor of the amino-acid-sensing pathway. Here, we find that the Sestrins, a family of poorly understood growth regulators (Sestrin1-Sestrin3), interact with GATOR2 in an amino-acid-sensitive fashion. Sestrin2-mediated inhibition of mTORC1 signaling requires GATOR1 and the Rag GTPases, and the Sestrins regulate the localization of mTORC1 in response to amino acids. Thus, we identify the Sestrins as GATOR2-interacting proteins that regulate the amino-acid-sensing branch of the mTORC1 pathway.
Copyright © 2014 The Authors. Published by Elsevier Inc. All rights reserved.
Figures
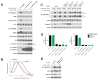
Figure 1. The Sestrins interact with GATOR2, but not GATOR1, in an amino acid-sensitive fashion
(A) GATOR2 interacts with the Sestrins. Mass spectrometric analyses identify Sestrinderived peptides in immunoprecipitates from HEK-293T cells stably expressing FLAGtagged GATOR2 components. (B) Recombinant Sestrin 1, 2, and 3 interact with recombinant GATOR2 but not GATOR1. Anti-FLAG immunoprecipitates were collected from HEK-293T cells expressing the indicated cDNAs in expression vectors and were analyzed, along with cell lysates, by immunoblotting for the relevant epitope tags. (C) Stably expressed Sestrin2 co-immunoprecipitates endogenous GATOR2 components. Immunoprecipitates were prepared from HEK-293T cells stably expressing the indicated FLAG-tagged proteins, and were analyzed along with cell lysates by immunoblotting for the indicated proteins. (D) Stably expressed GATOR2 and endogenous Sestrin2 interact in an amino acid dependent fashion. HEK-293T cells stably expressing the indicated FLAG-tagged proteins were starved of amino acids for 50 minutes, or starved and then stimulated with amino acids for 10 minutes. Anti-FLAG immunoprecipitates were analyzed as in (C). (E) Stably expressed Sestrin2 interacts with endogenous GATOR2 in an amino acidsensitive fashion. HEK-293T cells expressing the indicated epitope tagged proteins were amino acid starved or starved and restimulated with amino acids as in (D), and anti-FLAG immunoprecipitates were analyzed as in (C). (F) The GATOR2-Sestrin2 interaction is sensitive to both amino acid and glucose availability, but is not affected by growth factors. HEK-293T cells stably expressing the indicated FLAG-tagged proteins were starved of either amino acids, glucose, or growth factors for 50 minutes, or starved and restimulated with amino acids, glucose, or insulin, respectively, for 10 minutes. Anti-FLAG immunoprecipitates were analyzed as in (C).
Figure 2. The Sestrins are negative regulators of the amino acid sensing pathway upstream of mTORC1
(A) Stable overexpression of Sestrin2 inhibits mTORC1 signaling, but does not affect the phosphorylation of Akt. HEK-293T cells stably expressing the indicated proteins were starved of amino acids for 50 minutes, or starved and restimulated with amino acids for 10 minutes. Immunoblotting of cell lysates allowed for the analysis of levels and the phosphorylation states of the indicated proteins. (B) Stable overexpression of Sestrin2 severely decreased cell size. HEK-293T cells stably expressing the indicated proteins and wild-type HEK-293T cells were analyzed for cell size. (C) A decrease in the levels of the Sestrins leads to an inability to fully inhibit mTORC1 signaling under amino acid deprivation. HEK-293T cells which were genetically modified with the indicated guide RNAs using the CRISPR/Cas9 system were subsequently treated with the indicated shRNAs, then starved of amino acids for 50 minutes, or starved and restimulated with amino acids for 10 minutes, and analyzed as in (A). (D) The indicated shRNAs reduced the mRNA levels of Sestrin1 and 3. Quantitative polymerase chain reaction (qPCR) was performed on the samples described in (C) to assess the efficacy of shRNA-mediated knockdown of Sestrin1 and 3. Errors depicted are standard error of the mean calculated based on samples from a single qPCR run. (E) Double-knockdown of Sestrin1 and 2 exaggerates the observed phenotype. Cells were treated and cell lysates were analyzed as in (A).
Figure 3. The Sestrins function upstream of the Rag GTPases and GATOR1
(A) The Sestrins function upstream of the Rag GTPases. Rag heterodimers containing constitutively active RagB99L-RagC75N or the dominant negative RagB54N-RagC121L mutants were cotransfected alongside the indicated cDNAs in HEK-293T cells. Anti- FLAG immunoprecipitates were prepared and protein lysates were analyzed by immunoblotting for the indicated proteins. (B) GATOR1 is necessary for the Sestrins to inhibit mTORC1 signaling. The indicated constructs were stably overexpressed in control HEK-293E cells or in cells lacking the indicated GATOR1 components generated via the CRISPR/Cas9 method. Lysates were probed via immunoblotting for the indicated proteins.
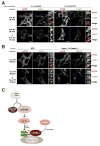
Figure 4. The Sestrins control mTORC1 localization in response to amino acids
(A) Sestrin2 overexpression prevents proper mTORC1 recruitment to lysosomes. HEK- 293T cells stably expressing the indicated recombinant proteins were starved or starved and restimulated with amino acids for the indicated times prior to processing for immunofluorescence. Insets depict selected fields that were magnified 3.24 times and their overlays. (B) Sestrin1 and Sestrin2 loss results in constitutive mTORC1 localization to the lysosome. HEK-293T cells stably expressing the indicated shRNA constructs were processed as described above in (A).
Similar articles
- Sestrins inhibit mTORC1 kinase activation through the GATOR complex.
Parmigiani A, Nourbakhsh A, Ding B, Wang W, Kim YC, Akopiants K, Guan KL, Karin M, Budanov AV. Parmigiani A, et al. Cell Rep. 2014 Nov 20;9(4):1281-91. doi: 10.1016/j.celrep.2014.10.019. Cell Rep. 2014. PMID: 25457612 Free PMC article. - A Tumor suppressor complex with GAP activity for the Rag GTPases that signal amino acid sufficiency to mTORC1.
Bar-Peled L, Chantranupong L, Cherniack AD, Chen WW, Ottina KA, Grabiner BC, Spear ED, Carter SL, Meyerson M, Sabatini DM. Bar-Peled L, et al. Science. 2013 May 31;340(6136):1100-6. doi: 10.1126/science.1232044. Science. 2013. PMID: 23723238 Free PMC article. - Sestrins function as guanine nucleotide dissociation inhibitors for Rag GTPases to control mTORC1 signaling.
Peng M, Yin N, Li MO. Peng M, et al. Cell. 2014 Sep 25;159(1):122-133. doi: 10.1016/j.cell.2014.08.038. Cell. 2014. PMID: 25259925 Free PMC article. - Regulation of mTORC1 by the Rag GTPases.
Lama-Sherpa TD, Jeong MH, Jewell JL. Lama-Sherpa TD, et al. Biochem Soc Trans. 2023 Apr 26;51(2):655-664. doi: 10.1042/BST20210038. Biochem Soc Trans. 2023. PMID: 36929165 Free PMC article. Review. - Rag GTPase in amino acid signaling.
Kim J, Kim E. Kim J, et al. Amino Acids. 2016 Apr;48(4):915-928. doi: 10.1007/s00726-016-2171-x. Epub 2016 Jan 18. Amino Acids. 2016. PMID: 26781224 Review.
Cited by
- Sending Mixed Signals: The Expanding Role of Molecular Cascade Mutations in Malformations of Cortical Development and Epilepsy.
Iffland PH 2nd, Crino PB. Iffland PH 2nd, et al. Epilepsy Curr. 2016 May-Jun;16(3):158-63. doi: 10.5698/1535-7511-16.3.158. Epilepsy Curr. 2016. PMID: 27330441 Free PMC article. - Lifelong restriction of dietary branched-chain amino acids has sex-specific benefits for frailty and lifespan in mice.
Richardson NE, Konon EN, Schuster HS, Mitchell AT, Boyle C, Rodgers AC, Finke M, Haider LR, Yu D, Flores V, Pak HH, Ahmad S, Ahmed S, Radcliff A, Wu J, Williams EM, Abdi L, Sherman DS, Hacker T, Lamming DW. Richardson NE, et al. Nat Aging. 2021 Jan;1(1):73-86. doi: 10.1038/s43587-020-00006-2. Epub 2021 Jan 14. Nat Aging. 2021. PMID: 33796866 Free PMC article. - Amino acids regulating skeletal muscle metabolism: mechanisms of action, physical training dosage recommendations and adverse effects.
Li G, Li Z, Liu J. Li G, et al. Nutr Metab (Lond). 2024 Jul 2;21(1):41. doi: 10.1186/s12986-024-00820-0. Nutr Metab (Lond). 2024. PMID: 38956658 Free PMC article. Review. - Novel Essential Amino Acid Supplements Following Resistance Exercise Induce Aminoacidemia and Enhance Anabolic Signaling Irrespective of Age: A Proof-of-Concept Trial.
Lees MJ, Wilson OJ, Webb EK, Traylor DA, Prior T, Elia A, Harlow PS, Black AD, Parker PJ, Harris N, Cooke M, Balchin C, Butterworth M, Phillips SM, Ispoglou T. Lees MJ, et al. Nutrients. 2020 Jul 12;12(7):2067. doi: 10.3390/nu12072067. Nutrients. 2020. PMID: 32664648 Free PMC article. - The Mechanistic Target of Rapamycin: The Grand ConducTOR of Metabolism and Aging.
Kennedy BK, Lamming DW. Kennedy BK, et al. Cell Metab. 2016 Jun 14;23(6):990-1003. doi: 10.1016/j.cmet.2016.05.009. Cell Metab. 2016. PMID: 27304501 Free PMC article. Review.
References
- Boussif O, Lezoualc’h F, Zanta MA, Mergny MD, Scherman D, Demeneix B, Behr JP. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:7297–7301. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32 GM007287/GM/NIGMS NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- T32 GM007753/GM/NIGMS NIH HHS/United States
- F31 CA180271/CA/NCI NIH HHS/United States
- R37 AI047389/AI/NIAID NIH HHS/United States
- R01 CA129105/CA/NCI NIH HHS/United States
- R01 GM067945/GM/NIGMS NIH HHS/United States
- AI47389/AI/NIAID NIH HHS/United States
- GM67945/GM/NIGMS NIH HHS/United States
- R01 AI047389/AI/NIAID NIH HHS/United States
- R01 CA103866/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous