α-synuclein multimers cluster synaptic vesicles and attenuate recycling - PubMed (original) (raw)
α-synuclein multimers cluster synaptic vesicles and attenuate recycling
Lina Wang et al. Curr Biol. 2014.
Abstract
The normal functions and pathologic facets of the small presynaptic protein α-synuclein (α-syn) are of exceptional interest. In previous studies, we found that α-syn attenuates synaptic exo/endocytosis; however, underlying mechanisms remain unknown. More recent evidence suggests that α-syn exists as metastable multimers and not solely as a natively unfolded monomer. However, conformations of α-syn at synapses--its physiologic locale--are unclear, and potential implications of such higher-order conformations to synaptic function are unknown. Exploring α-syn conformations and synaptic function in neurons, we found that α-syn promptly organizes into physiological multimers at synapses. Furthermore, our experiments indicate that α-syn multimers cluster synaptic vesicles and restrict their motility, suggesting a novel role for these higher-order structures. Supporting this, α-syn mutations that disrupt multimerization also fail to restrict synaptic vesicle motility or attenuate exo/endocytosis. We propose a model in which α-syn multimers cluster synaptic vesicles, restricting their trafficking and recycling, and consequently attenuate neurotransmitter release.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Figures
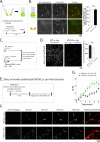
Figure 1. Multimeric α-syn conformations at presynaptic boutons
(A) Schematic of complementation assay (i) and molecular replacement strategy (ii). Cultured hippocampal neurons from α-syn −/− mice were transiently transfected with various VN/VC-tagged α-syn's (see “results”) and visualized after ~ 14 hours. (B)
Top
: Representative images of reconstituted Venus fluorescence in neurons expressing VN/VC:α-syn's (also see Supp. fig. 1A). Note these neurons are co-transfected with synaptophysin:mRFP (SyPhy:mRFP) to label boutons.
Bottom
: No fluorescence was seen in boutons expressing un-tagged VN + VC alone.
Right
: The vast majority (~85%) of SyPhy:mRFP-positive boutons also expressed VN/VC:α-syn; comparable to boutons expressing Venus:α-syn and SyPhy:mRFP (N~700 boutons for each group from two separate batches of cultures, p=0.90). (C) Overall design to compare expression-levels of transfected VN/VC:α-syn to endogenous mouse α-syn. Un-transfected cultured neurons from WT mice and VN/VC:α-syn-transfected cultured neurons from α-syn −/− mice were fixed and immunostained with an anti-α-syn antibody (guinea-pig α-syn antibody). Cell culture and immunostaining of both groups were processed in parallel. Note that while the antibody would recognize mouse α-syn in WT neurons, it would only label transfected α-syn in the VN/VC:α-syn transfected group. (D) Representative images from the two groups in (C) (left) and quantification of overall average fluorescence intensities (right; N~10 visual fields containing ~ 3000-10,000 boutons; p=0.06). Note that the number of VN/VC:α-syn transfected boutons is much lower than immunostained WT boutons (as expected with transient transfections), but the fluorescence-intensities are similar. (E) Overall design. Cultured α-syn −/− neurons were co-transfected with VN/VC:α-syn's (or Venus:α-syn) + soluble mCherry, and kinetics of initial α-syn entry and synaptic accumulation was evaluated by long-term imaging (see “results” and [21] for more details). (F) Representative frames from two time-lapse movies showing pre-synaptic accumulation of VN/VC:α-syn (top) and Venus:α-syn (bottom) over 5 hrs of imaging. (G) Quantification of average VFP intensities of boutons over 5 hrs. Note that though the kinetics of VN/VC:α-syn accumulation (black dots) is slower than Venus:α-syn (green dots) as expected, the difference is modest, suggesting that complementation is a relatively early event.
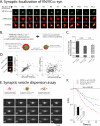
Figure 2. α-syn multimers cluster synaptic-vesicles
(A) Bouton-crops from neurons co-transfected with VN/VC:α-syn and mRFP:Actin (to label entire bouton-profile, see “results”). Note that reconstituted VN/VC:α-syn's only occupy a fraction of the bouton cross-sectional area. (B) Experimental design: Neurons were co-transfected with VN/VC:α-syn and markers to label the entire bouton-profile (mRFP:Actin) or synaptic-vesicles (SyPhy:mRFP); and extent of overlap was determined by custom algorithms (see “results” and “methods” for details). (C, D) Both reconstituted VN/VC:α-syn and SyPhy:GFP occupied a smaller fraction of the bouton than Venus:α-syn (~ 200 boutons analyzed for each group from two separate batches of cultures, ***p < 0.001). (D) Bouton-widths (FWHM, see methods) of VN/VC:α-syn and SyPhy:mRFP were correlated (left; r=0.36, p<0.0001), unlike VN/VC:α-syn and mRFP:Actin, further indicating associations of complemented VN/VC:α-syn's with synaptic-vesicles (N=120 boutons from two separate batches of cultures). (E)
Top
: Schematic of “synaptic-vesicle dispersion assay”. Synaptic-vesicles are labeled by SyPhy:mRFP and neurons are stimulated to disperse synaptic-vesicles (see “results”).
Bottom
: A time-series showing dispersion of synaptic-vesicles from a bouton (elapsed time in seconds on lower left, asterisk marks the start of stimulation). (F) Quantification of synaptic-vesicle dispersion using above assay. While Venus:α-syn diminishes dispersion-kinetics (compared to vector), the dispersion is further attenuated by VN/VC:α-syn (note that error bars are too small to be seen). Extent of dispersion quantified in inset (19.5%, 13.6% and 9% of total synaptic-vesicles were dispersed in vector, Venus:α-syn and VN/VC:α-syn groups respectively; ***p < 0.001, unpaired t test).
Figure 3. Biochemical analyses of α-syn multimers
(A) VN/VC:α-syn's were introduced into HEK293T cells or neurons (by viruses), expressed for the times indicated, and cell-lysates were analyzed by Native/SDS-PAGE. (B) Native-PAGE show α-syn higher-order multimers immunoblotted with two α-syn antibodies and an anti-GFP antibody that also recognizes YFP (note disruption upon boiling). The red arrow marks the position where bands are typically seen, black arrow marks putative monomeric α-syn in neurons. An SDS-PAGE immunoblotted with anti-GFP marks the VFP-fragments. Each experiment was repeated 3-5 times with similar results. (C) In-vitro reconstitution assay. Purified synaptic-vesicles and cytosol from α-syn −/− mouse brains were mixed with WT-α-syn purified from bacteria with/without a chemical cross linker (DSG). Vesicle membrane bound and unbound fractions were separated by centrifugation and analyzed by SDS-PAGE. (D) Both monomeric and cross-linked α-syn multimers bound to synaptic-vesicles (a synaptophysin stain confirms that all synaptic-vesicles are in the bound fraction). Red and black arrows mark positions of putative tetramers and monomers. Experiment was repeated twice with similar results.
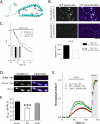
Figure 4. Mechanistic links between α-syn multimerization and synaptic function
(A) Schematic of the α-syn helices (shaded) and position of the six mutations. (B) Neurons from α-syn −/− mice were transfected with VN/VC:WT or VN/VC:TsixK α-syn's and fluorescence was quantified in boutons. There were clear diminutions in the TsixK datasets as shown in the representative images and quantification below. (C) “Synaptic-vesicle dispersion” assay: Neurons were co-transfected with SyPhy:mRFP (to label synaptic-vesicles) and untagged WT or TsixK α-syn (or vector alone). Boutons were stimulated and decay of RFP fluorescence from boutons was quantified (see “results”). Note that while WT α-syn attenuates activity-induced synaptic-vesicle dispersion, the TsixK mutant has no effect on vesicle-trafficking (N=number of boutons). (D) Synaptic recycling evaluated by vGlut-pHluorin assays. Cultured neurons were co transfected with vGlut-pHluorin and either untagged WT α-syn or TsixK α-syn. Fluorescence-change of the pH-sensitive vGlut-pHluorin probe reflects synaptic-vesicle recycling in this assay (see “results” and “methods”). Representative panels show fluorescence intensity change of vGlut-pHluorin upon 600 AP stimulation and NH4Cl perfusion. Note that NH4Cl alkalinizes all vesicles, revealing the total (recycling + resting) pool in these neurons. (E, F) Representative ensemble average of vGlut-pHluorin traces from empty vector, WT α-syn or TsixK α-syn transfected neurons (N=number of boutons). Note that while WT α-syn nattenuates neurotransmitter release and decreases mean recycling-pools compared to vector-controls, TsixK α-syn fails to show this effect; quantified in (F) (all data normalized to total pools). Recycling/total pool for vector=43±2.17 %; WT α-syn =28±2.38%; TsixK α-syn =39±2.29% (~ 160 boutons on 7-9 coverslips were analyzed for each group from three separate batches of cultures; ***p < 0.001 compared to vector by one-way ANOVA followed by Dunnet's post hoc test). Total (alkalinized) pools of vector, WT-α-syn and TsixK-α-syn groups were 317.1 ± 16 AFU, 317.5 ± 11 AFU and 376 ± 18 AFU (mean ± SEM) respectively).
Similar articles
- Synapsins regulate α-synuclein functions.
Atias M, Tevet Y, Sun J, Stavsky A, Tal S, Kahn J, Roy S, Gitler D. Atias M, et al. Proc Natl Acad Sci U S A. 2019 Jun 4;116(23):11116-11118. doi: 10.1073/pnas.1903054116. Epub 2019 May 20. Proc Natl Acad Sci U S A. 2019. PMID: 31110014 Free PMC article. - Functional cooperation of α-synuclein and VAMP2 in synaptic vesicle recycling.
Sun J, Wang L, Bao H, Premi S, Das U, Chapman ER, Roy S. Sun J, et al. Proc Natl Acad Sci U S A. 2019 Jun 4;116(23):11113-11115. doi: 10.1073/pnas.1903049116. Epub 2019 May 20. Proc Natl Acad Sci U S A. 2019. PMID: 31110017 Free PMC article. - Synapsin E-domain is essential for α-synuclein function.
Stavsky A, Parra-Rivas LA, Tal S, Riba J, Madhivanan K, Roy S, Gitler D. Stavsky A, et al. Elife. 2024 May 7;12:RP89687. doi: 10.7554/eLife.89687. Elife. 2024. PMID: 38713200 Free PMC article. - The Role of α-Synuclein in SNARE-mediated Synaptic Vesicle Fusion.
Yoo G, Shin YK, Lee NK. Yoo G, et al. J Mol Biol. 2023 Jan 15;435(1):167775. doi: 10.1016/j.jmb.2022.167775. Epub 2022 Aug 3. J Mol Biol. 2023. PMID: 35931109 Review. - The role of α-synuclein in neurotransmission and synaptic plasticity.
Cheng F, Vivacqua G, Yu S. Cheng F, et al. J Chem Neuroanat. 2011 Dec;42(4):242-8. doi: 10.1016/j.jchemneu.2010.12.001. Epub 2010 Dec 16. J Chem Neuroanat. 2011. PMID: 21167933 Review.
Cited by
- A novel multiplex assay for simultaneous quantification of total and S129 phosphorylated human alpha-synuclein.
Landeck N, Hall H, Ardah MT, Majbour NK, El-Agnaf OM, Halliday G, Kirik D. Landeck N, et al. Mol Neurodegener. 2016 Aug 22;11(1):61. doi: 10.1186/s13024-016-0125-0. Mol Neurodegener. 2016. PMID: 27549140 Free PMC article. - Genes Implicated in Familial Parkinson's Disease Provide a Dual Picture of Nigral Dopaminergic Neurodegeneration with Mitochondria Taking Center Stage.
Franco R, Rivas-Santisteban R, Navarro G, Pinna A, Reyes-Resina I. Franco R, et al. Int J Mol Sci. 2021 Apr 28;22(9):4643. doi: 10.3390/ijms22094643. Int J Mol Sci. 2021. PMID: 33924963 Free PMC article. Review. - Neurodevelopmental and synaptic defects in DNAJC6 parkinsonism, amenable to gene therapy.
Abela L, Gianfrancesco L, Tagliatti E, Rossignoli G, Barwick K, Zourray C, Reid KM, Budinger D, Ng J, Counsell J, Simpson A, Pearson TS, Edvardson S, Elpeleg O, Brodsky FM, Lignani G, Barral S, Kurian MA. Abela L, et al. Brain. 2024 Jun 3;147(6):2023-2037. doi: 10.1093/brain/awae020. Brain. 2024. PMID: 38242634 Free PMC article. - Serine-129 phosphorylation of α-synuclein is an activity-dependent trigger for physiologic protein-protein interactions and synaptic function.
Parra-Rivas LA, Madhivanan K, Aulston BD, Wang L, Prakashchand DD, Boyer NP, Saia-Cereda VM, Branes-Guerrero K, Pizzo DP, Bagchi P, Sundar VS, Tang Y, Das U, Scott DA, Rangamani P, Ogawa Y, Subhojit Roy. Parra-Rivas LA, et al. Neuron. 2023 Dec 20;111(24):4006-4023.e10. doi: 10.1016/j.neuron.2023.11.020. Neuron. 2023. PMID: 38128479 Free PMC article. - The effects of KTKEGV repeat motif and intervening ATVA sequence on α-synuclein solubility and assembly.
Brontesi L, Imberdis T, Ramalingam N, Dettmer U. Brontesi L, et al. J Neurochem. 2023 Apr;165(2):246-258. doi: 10.1111/jnc.15763. Epub 2023 Jan 28. J Neurochem. 2023. PMID: 36625497 Free PMC article.
References
- Larsen KE, Schmitz Y, Troyer MD, Mosharov E, Dietrich P, Quazi AZ, Savalle M, Nemani V, Chaudhry FA, Edwards RH, et al. Alpha-synuclein overexpression in PC12 and chromaffin cells impairs catecholamine release by interfering with a late step in exocytosis. J Neurosci. 2006;26:11915–11922. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous