Crizotinib in ROS1-rearranged non-small-cell lung cancer - PubMed (original) (raw)
Clinical Trial
. 2014 Nov 20;371(21):1963-71.
doi: 10.1056/NEJMoa1406766. Epub 2014 Sep 27.
Sai-Hong I Ou, Yung-Jue Bang, D Ross Camidge, Benjamin J Solomon, Ravi Salgia, Gregory J Riely, Marileila Varella-Garcia, Geoffrey I Shapiro, Daniel B Costa, Robert C Doebele, Long Phi Le, Zongli Zheng, Weiwei Tan, Patricia Stephenson, S Martin Shreeve, Lesley M Tye, James G Christensen, Keith D Wilner, Jeffrey W Clark, A John Iafrate
Affiliations
- PMID: 25264305
- PMCID: PMC4264527
- DOI: 10.1056/NEJMoa1406766
Clinical Trial
Crizotinib in ROS1-rearranged non-small-cell lung cancer
Alice T Shaw et al. N Engl J Med. 2014.
Abstract
Background: Chromosomal rearrangements of the gene encoding ROS1 proto-oncogene receptor tyrosine kinase (ROS1) define a distinct molecular subgroup of non-small-cell lung cancers (NSCLCs) that may be susceptible to therapeutic ROS1 kinase inhibition. Crizotinib is a small-molecule tyrosine kinase inhibitor of anaplastic lymphoma kinase (ALK), ROS1, and another proto-oncogene receptor tyrosine kinase, MET.
Methods: We enrolled 50 patients with advanced NSCLC who tested positive for ROS1 rearrangement in an expansion cohort of the phase 1 study of crizotinib. Patients were treated with crizotinib at the standard oral dose of 250 mg twice daily and assessed for safety, pharmacokinetics, and response to therapy. ROS1 fusion partners were identified with the use of next-generation sequencing or reverse-transcriptase-polymerase-chain-reaction assays.
Results: The objective response rate was 72% (95% confidence interval [CI], 58 to 84), with 3 complete responses and 33 partial responses. The median duration of response was 17.6 months (95% CI, 14.5 to not reached). Median progression-free survival was 19.2 months (95% CI, 14.4 to not reached), with 25 patients (50%) still in follow-up for progression. Among 30 tumors that were tested, we identified 7 ROS1 fusion partners: 5 known and 2 novel partner genes. No correlation was observed between the type of ROS1 rearrangement and the clinical response to crizotinib. The safety profile of crizotinib was similar to that seen in patients with ALK-rearranged NSCLC.
Conclusions: In this study, crizotinib showed marked antitumor activity in patients with advanced ROS1-rearranged NSCLC. ROS1 rearrangement defines a second molecular subgroup of NSCLC for which crizotinib is highly active. (Funded by Pfizer and others; ClinicalTrials.gov number, NCT00585195.).
Figures
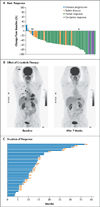
Figure 1. Tumor Responses to Crizotinib in _ROS1_-Rearranged Non–Small-Cell Lung Cancer
Panel A shows the best response of patients treated with crizotinib in the ROS1 expansion cohort. The bars indicate best percent change in the target tumor burden from baseline. Two patients died within 6 weeks after receiving the first dose of crizotinib, so the tumor response was unknown. The asterisk indicates the tumor burden in a patient who had an atypical result on fluorescence in situ hybridization (FISH) for ROS1 (an isolated 5′ green signal). Since this tumor was subsequently shown to be negative for ROS1 rearrangement on next-generation sequencing, an isolated green signal is probably not indicative of a ROS1 rearrangement. The letter A denotes a FISH-positive tumor that was negative for ROS1 rearrangement on next-generation sequencing but positive for ALK rearrangement on FISH and next-generation sequencing. The letter M denotes a FISH-positive tumor that was also positive for MET amplification on FISH (_MET_-to-CEP7 ratio, 5.34). Panel B shows positron-emission tomographic scans obtained at baseline (left panel) and after 7 weeks of crizotinib treatment (right panel) in a representative patient. On the basis of Response Evaluation Criteria in Solid Tumors, this patient had a partial response (a decrease in tumor burden of 46%), which was ongoing at the time of data cutoff. Panel C shows the duration of response among the 36 patients with a partial or complete response. Arrows indicate patients who had an ongoing response at the time of data cutoff. The letter A indicates that the patient’s tumor was positive for ALK rearrangement.
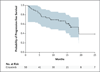
Figure 2. Progression-free Survival
Shown is the Kaplan–Meier curve for estimated progression-free survival in the ROS1 cohort of patients treated with crizotinib. Progression-free survival was defined as the time from the administration of the first dose of crizotinib to objective disease progression or death from any cause. Data from 27 patients were censored; of these patients, 25 remained in follow-up for progression-free survival at the time of data cutoff. The shaded area represents the 95% Hall–Wellner confidence limits. Vertical lines on the survival curve indicate censoring of data.
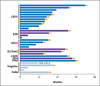
Figure 3. Duration of Treatment and ROS1 Fusion Partners
The duration of crizotinib treatment is shown for the 25 patients in whom the ROS1 fusion partner was identified with the use of either a next-generation sequencing assay or a reverse-transcriptase–polymerase-chain-reaction assay. Patients are grouped according to the ROS1 fusion partner, as indicated on the left. The four patients with negative results on next-generation sequencing and the one patient in whom next-generation sequencing failed are indicated by gray bars. One of the four patients with negative results was positive for EML4-ALK rearrangement, as indicated. One patient had negative results on next-generation sequencing and had an atypical FISH pattern (as indicated by an asterisk). The arrows indicate patients who were continuing to receive crizotinib at the time of data cutoff.
Comment in
- Lung cancer: alternative rearrangements--targeting ROS1 in NSCLC.
Killock D. Killock D. Nat Rev Clin Oncol. 2014 Nov;11(11):624. doi: 10.1038/nrclinonc.2014.180. Epub 2014 Oct 14. Nat Rev Clin Oncol. 2014. PMID: 25311353 No abstract available. - ROS1--targeting the one percent in lung cancer.
Gold KA. Gold KA. N Engl J Med. 2014 Nov 20;371(21):2030-1. doi: 10.1056/NEJMe1411319. N Engl J Med. 2014. PMID: 25409376 No abstract available. - Crizotinib in ROS1-rearranged non-small-cell lung cancer.
Shaw AT, Solomon BJ. Shaw AT, et al. N Engl J Med. 2015 Feb 12;372(7):683-4. doi: 10.1056/NEJMc1415359. N Engl J Med. 2015. PMID: 25671264 No abstract available. - Crizotinib in ROS1-rearranged non-small-cell lung cancer.
Arnaoutakis K. Arnaoutakis K. N Engl J Med. 2015 Feb 12;372(7):683. doi: 10.1056/NEJMc1415359. N Engl J Med. 2015. PMID: 25671265 No abstract available.
Similar articles
- ROS1 rearrangements define a unique molecular class of lung cancers.
Bergethon K, Shaw AT, Ou SH, Katayama R, Lovly CM, McDonald NT, Massion PP, Siwak-Tapp C, Gonzalez A, Fang R, Mark EJ, Batten JM, Chen H, Wilner KD, Kwak EL, Clark JW, Carbone DP, Ji H, Engelman JA, Mino-Kenudson M, Pao W, Iafrate AJ. Bergethon K, et al. J Clin Oncol. 2012 Mar 10;30(8):863-70. doi: 10.1200/JCO.2011.35.6345. Epub 2012 Jan 3. J Clin Oncol. 2012. PMID: 22215748 Free PMC article. - Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer.
Kwak EL, Bang YJ, Camidge DR, Shaw AT, Solomon B, Maki RG, Ou SH, Dezube BJ, Jänne PA, Costa DB, Varella-Garcia M, Kim WH, Lynch TJ, Fidias P, Stubbs H, Engelman JA, Sequist LV, Tan W, Gandhi L, Mino-Kenudson M, Wei GC, Shreeve SM, Ratain MJ, Settleman J, Christensen JG, Haber DA, Wilner K, Salgia R, Shapiro GI, Clark JW, Iafrate AJ. Kwak EL, et al. N Engl J Med. 2010 Oct 28;363(18):1693-703. doi: 10.1056/NEJMoa1006448. N Engl J Med. 2010. PMID: 20979469 Free PMC article. Clinical Trial. - Clinical features of Bim deletion polymorphism and its relation with crizotinib primary resistance in Chinese patients with ALK/ROS1 fusion-positive non-small cell lung cancer.
Zhang L, Jiang T, Li X, Wang Y, Zhao C, Zhao S, Xi L, Zhang S, Liu X, Jia Y, Yang H, Shi J, Su C, Ren S, Zhou C. Zhang L, et al. Cancer. 2017 Aug 1;123(15):2927-2935. doi: 10.1002/cncr.30677. Epub 2017 Mar 27. Cancer. 2017. PMID: 28346673 - Crizotinib for the treatment of ALK-rearranged non-small cell lung cancer: a success story to usher in the second decade of molecular targeted therapy in oncology.
Ou SH, Bartlett CH, Mino-Kenudson M, Cui J, Iafrate AJ. Ou SH, et al. Oncologist. 2012;17(11):1351-75. doi: 10.1634/theoncologist.2012-0311. Epub 2012 Sep 18. Oncologist. 2012. PMID: 22989574 Free PMC article. Review. - Targeted therapies in non-small cell lung cancer: a focus on ALK/ROS1 tyrosine kinase inhibitors.
Sgambato A, Casaluce F, Maione P, Gridelli C. Sgambato A, et al. Expert Rev Anticancer Ther. 2018 Jan;18(1):71-80. doi: 10.1080/14737140.2018.1412260. Epub 2017 Dec 6. Expert Rev Anticancer Ther. 2018. PMID: 29187012 Review.
Cited by
- Approach to biomarker testing: perspectives from various specialties.
Sung MR, Ellis PM, Verma S, Duncan E, Leighl NB. Sung MR, et al. Curr Oncol. 2016 Jun;23(3):178-83. doi: 10.3747/co.23.3019. Epub 2016 Jun 9. Curr Oncol. 2016. PMID: 27330346 Free PMC article. - Non-Small Cell Lung Cancer from Genomics to Therapeutics: A Framework for Community Practice Integration to Arrive at Personalized Therapy Strategies.
Rajurkar S, Mambetsariev I, Pharaon R, Leach B, Tan T, Kulkarni P, Salgia R. Rajurkar S, et al. J Clin Med. 2020 Jun 15;9(6):1870. doi: 10.3390/jcm9061870. J Clin Med. 2020. PMID: 32549358 Free PMC article. Review. - Clinical impact of a cancer genomic profiling test using an in-house comprehensive targeted sequencing system.
Hayashi H, Tanishima S, Fujii K, Mori R, Okada C, Yanagita E, Shibata Y, Matsuoka R, Amano T, Yamada T, Yabe I, Kinoshita I, Komatsu Y, Dosaka-Akita H, Nishihara H. Hayashi H, et al. Cancer Sci. 2020 Oct;111(10):3926-3937. doi: 10.1111/cas.14608. Epub 2020 Sep 6. Cancer Sci. 2020. PMID: 32772458 Free PMC article. - An Oncogenic NTRK Fusion in a Patient with Soft-Tissue Sarcoma with Response to the Tropomyosin-Related Kinase Inhibitor LOXO-101.
Doebele RC, Davis LE, Vaishnavi A, Le AT, Estrada-Bernal A, Keysar S, Jimeno A, Varella-Garcia M, Aisner DL, Li Y, Stephens PJ, Morosini D, Tuch BB, Fernandes M, Nanda N, Low JA. Doebele RC, et al. Cancer Discov. 2015 Oct;5(10):1049-57. doi: 10.1158/2159-8290.CD-15-0443. Epub 2015 Jul 27. Cancer Discov. 2015. PMID: 26216294 Free PMC article. Clinical Trial. - Necitumumab plus gemcitabine and cisplatin in previously treated lung squamous cell carcinoma.
Kinoshita F, Oku Y, Takamori S, Fujishita T, Toyozawa R, Ito K, Shoji F, Okamoto T. Kinoshita F, et al. Invest New Drugs. 2023 Feb;41(1):168-172. doi: 10.1007/s10637-022-01312-9. Epub 2022 Nov 4. Invest New Drugs. 2023. PMID: 36331673
References
- Acquaviva J, Wong R, Charest A. The multifaceted roles of the receptor tyrosine kinase ROS in development and cancer. Biochim Biophys Acta. 2009;1795:37–52. - PubMed
- Charest A, Lane K, McMahon K, et al. Fusion of FIG to the receptor tyrosine kinase ROS in a glioblastoma with an interstitial del(6)(q21q21) Genes Chromosomes Cancer. 2003;37:58–71. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30CA046934/CA/NCI NIH HHS/United States
- P50CA058187/CA/NCI NIH HHS/United States
- R21CA161590/CA/NCI NIH HHS/United States
- R21 CA161590/CA/NCI NIH HHS/United States
- K12 CA086913/CA/NCI NIH HHS/United States
- P50 CA058187/CA/NCI NIH HHS/United States
- K12CA086913/CA/NCI NIH HHS/United States
- P30 CA008748/CA/NCI NIH HHS/United States
- P30 CA046934/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous