Origins and functional consequences of somatic mitochondrial DNA mutations in human cancer - PubMed (original) (raw)
doi: 10.7554/eLife.02935.
Ludmil B Alexandrov 1, Moritz Gerstung 1, Inigo Martincorena 1, Serena Nik-Zainal 1, Manasa Ramakrishna 1, Helen R Davies 1, Elli Papaemmanuil 1, Gunes Gundem 1, Adam Shlien 1, Niccolo Bolli 1, Sam Behjati 1, Patrick S Tarpey 1, Jyoti Nangalia 1, Charles E Massie 1, Adam P Butler 1, Jon W Teague 1, George S Vassiliou 1, Anthony R Green 2, Ming-Qing Du 2, Ashwin Unnikrishnan 3, John E Pimanda 3, Bin Tean Teh 4, Nikhil Munshi 5, Mel Greaves 6, Paresh Vyas 7, Adel K El-Naggar 8, Tom Santarius 2, V Peter Collins 2, Richard Grundy 9, Jack A Taylor 10, D Neil Hayes 11, David Malkin 12; ICGC Breast Cancer Group; ICGC Chronic Myeloid Disorders Group; ICGC Prostate Cancer Group; Christopher S Foster 13, Anne Y Warren 2, Hayley C Whitaker 14, Daniel Brewer 6, Rosalind Eeles 6, Colin Cooper 6, David Neal 14, Tapio Visakorpi 15, William B Isaacs 16, G Steven Bova 15, Adrienne M Flanagan 17, P Andrew Futreal 1, Andy G Lynch 14, Patrick F Chinnery 18, Ultan McDermott 1, Michael R Stratton 1, Peter J Campbell 1
Affiliations
- PMID: 25271376
- PMCID: PMC4371858
- DOI: 10.7554/eLife.02935
Origins and functional consequences of somatic mitochondrial DNA mutations in human cancer
Young Seok Ju et al. Elife. 2014.
Abstract
Recent sequencing studies have extensively explored the somatic alterations present in the nuclear genomes of cancers. Although mitochondria control energy metabolism and apoptosis, the origins and impact of cancer-associated mutations in mtDNA are unclear. In this study, we analyzed somatic alterations in mtDNA from 1675 tumors. We identified 1907 somatic substitutions, which exhibited dramatic replicative strand bias, predominantly C > T and A > G on the mitochondrial heavy strand. This strand-asymmetric signature differs from those found in nuclear cancer genomes but matches the inferred germline process shaping primate mtDNA sequence content. A number of mtDNA mutations showed considerable heterogeneity across tumor types. Missense mutations were selectively neutral and often gradually drifted towards homoplasmy over time. In contrast, mutations resulting in protein truncation undergo negative selection and were almost exclusively heteroplasmic. Our findings indicate that the endogenous mutational mechanism has far greater impact than any other external mutagens in mitochondria and is fundamentally linked to mtDNA replication.
Keywords: cancer genome; evolution; evolutionary biology; genomics; human; mitochondrial DNA; mutational signature; sequencing; somatic mutation.
Conflict of interest statement
The authors declare that no competing interests exist.
Figures
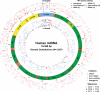
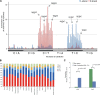
Figure 1.. Mitochondrial somatic substitutions identified from 1675 Tumor–Normal pairs.
mtDNA genes and intergenic regions are shown. The strand of genes is shown based on mtDNA strand containing equivalent sequences of transcribed RNA. Substitution categories (silent, non-silent (missense and nonsense), non-coding (tRNA and rRNA), and intergenic) are shown by the shapes of each substitution. Six classes of substitutions are presented color-coded. The substitutions on the H, and L strand (when six substitutional classes were considered) are shown outside and inside of mtDNA genes, respectively. Vertical axes for H and L strand substitutions represent the VAF of each variant. DOI:
http://dx.doi.org/10.7554/eLife.02935.004
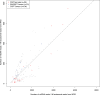
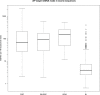
Figure 1—figure supplement 1.. Correlation in amount of mtDNA reads between whole-genome and whole-exome sequencing.
139 DNA samples, either from tumors or bloods, sequenced by whole-genome sequencing were additionally sequenced by whole-exome sequencing. We compared the amount of mtDNA reads between whole-genome and whole-exome sequencing. As shown in this figure, we found strong positive correlation. * CGP; Cancer Genome Project, Wellcome Trust Sanger Institute, WUGSC; Washington University Genome Sequencing Center. DOI:
http://dx.doi.org/10.7554/eLife.02935.005
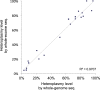
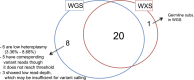
Figure 1—figure supplement 2.. Correlation of heteroplasmy levels between whole-genome and whole-exome sequencing.
To validate the sensitivity and specificity of variant calling in this study, 19 tumor and normal pairs (which were originally whole-genome sequenced) were whole-exome sequenced and mtDNA variants were assessed independently. We correlated the heteroplasmic levels of 20 mutations detected in common. DOI:
http://dx.doi.org/10.7554/eLife.02935.006
Figure 1—figure supplement 3.. Validation of mtDNA somatic substitutions.
DOI:
http://dx.doi.org/10.7554/eLife.02935.007
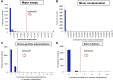
Figure 1—figure supplement 4.. Amount of off-target mtDNA reads across four sequencing centers.
* CGP; Cancer Genome Project, Wellcome Trust Sanger Institute (n = 855), WUGSC; Washington University Genome Sequencing Center (n = 140), BCM; Baylor College of Medicine (n = 85), BI; Broad Institute (n = 435). DOI:
http://dx.doi.org/10.7554/eLife.02935.008
Figure 1—figure supplement 5.. Filtering samples of potential DNA contaminations.
(A) A histogram presenting potential sample swaps in tumor–sample pairs. (B) A histogram presenting potential minor DNA cross-contamination in tumor samples. Cross-contamination levels were considered in filtering substitutions (see “Minor cross-contamination of DNA samples” section in Materials and Methods). (C) Histograms showing number of somatic substitutions overlapping with known inherited polymorphisms and (D) number of back mutations. DOI:
http://dx.doi.org/10.7554/eLife.02935.009
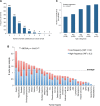
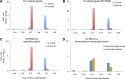
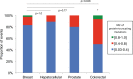
Figure 2.. mtDNA somatic substitutions of human cancer.
(A) Number of somatic substitutions in a tumor sample. (B) Average number of somatic substitutions per sample across 31 tumor types. (C) Age of diagnosis and number of mtDNA somatic substitutions in breast cancers. DOI:
http://dx.doi.org/10.7554/eLife.02935.010
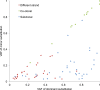
Figure 2—figure supplement 1.. VAFs of phased somatic mtDNA substitutions.
This figure presents VAF pairs between co-clonal, sub-clonal, and different strand mtDNA substitutions. We expect similar VAFs for co-clonal pairs; lower VAF in sub-clonal mutations compared to clonal ones; and sum of a VAF pair is equal or less than 1.0. DOI:
http://dx.doi.org/10.7554/eLife.02935.011
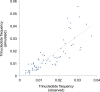
Figure 3.. Replicative strand bias for mtDNA somatic substitutions.
(A) Replicative strand-specific substitution rate (# of observed/# of expected) by 96 trinucleotide context. Substitutions in a specific mtDNA segment (from Ori-b to OH) are not included, because they present a different substitutional signature. (B) Mutational signature across tumor types. Eighteen tumor types, which include at least 25 mtDNA mutations, were shown. (C) Inverted substitution signature in the Ori-b–OH. DOI:
http://dx.doi.org/10.7554/eLife.02935.012
Figure 3—figure supplement 1.. Replicative strand bias observed in mtDNA substitutions.
(A) Mutational signature of mtDNA somatic substitutions on the 12 L strand genes by replicative strand (L/H strand). It agrees very well with the background mutational signature. (Chi-square p = 0.99999). (B) Mutational signature of mtDNA somatic substitutions on the H strand gene (MT-ND6) by replicative strand. It is very close to the background very close to the expected background signature (Chi-square p = 0.027). If we consider signature by transcriptional strand, the signature difference is very clear (Chi-square p = 1 × 10−21). These suggest the strand bias not to be transcription-coupled, but replication coupled. (C) Mutational spectrum of mtDNA somatic substitutions on the 22 tRNA genes by replicative strand. Again, it agrees very well with the background mutational signature (Chi-square p = 0.71). (D) Mutational spectrum of mtDNA somatic substitutions on the 22 tRNA genes by non-transcribed (coding) and transcribed (non-coding) strand. Strand bias was greatly subsided because somatic substitutions on 14 L strand and 8 H strand tRNAs neutralize the strand bias (CH > THand TL > CL) each other. As a result, this signature of tRNA mutations by transcriptional strand is significantly different from the background one (Chi-square p = 3.3 × 10−12). Taken all together, we concluded that the cause of strand bias is not transcription-coupled but is replicative. DOI:
http://dx.doi.org/10.7554/eLife.02935.013
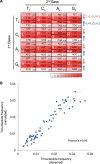
Figure 4.. Mutational signature similar to processes shaping human mtDNA sequence over evolutionary time.
(A) Triplet codon depletion in human mtDNA by equivalent (CH > TH and TL > CL) mutational pressure. Relative frequency of each triplet codon within synonymous pairs (NNT–NNC or NNA–NNG) is shown by color. The arrows beside the box highlight the T > C (red) and G > A (blue) substitutional pressures on the L strand in germline mtDNA. (B) Correlation of triplet codon frequencies between from observed and from simulated evolutions of a random sequence mtDNA by the mtDNA somatic mutational signature with constraining mitochondrial protein sequences. DOI:
http://dx.doi.org/10.7554/eLife.02935.014
Figure 4—figure supplement 1.. TC and GA skew for L strand mtDNA genes across 8 animal species.
C. elegans (a nematode) and D. melanogaster (fruit fly) mtDNA appears to have GL<< AL (due to CH > THmutational pressure) and CL >> TL (due to CL > TL mutational pressure) in the third base of triplet codon in L strand genes. Therefore they seem to have predominant C > T mutational pressure without strand bias. D. rerio (zebrafish), X. laevis (frog), and_M. musculus_ (mouse) present GL << AL (due to CH > TH mutational pressure), but similar number of CL and TL. Therefore, mtDNA of these sequences is thought to have CH > TH, with strand bias. The existence of TL > CL is not clear. Finally, mtDNA of H. sapiens, P. troglodytes (Chimpanzee), and_G. domesticus_ (Chicken) shows clear CH> TH and TL > CL as mentioned in the main manuscript. Interestingly, TL > CLseems to be slightly stronger in the mitochondria of chicken than that of human (or chimp). We suggest there would be some differences in the mechanism of mtDNA replication across the evolution tree. DOI:
http://dx.doi.org/10.7554/eLife.02935.015
Figure 4—figure supplement 2.. Correlation of triplet codon frequencies between from observed and from simulated evolutions under the mtDNA somatic mutational signature.
DOI:
http://dx.doi.org/10.7554/eLife.02935.016
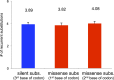
Figure 5.. Selection and mutational process for mtDNA somatic substitutions.
(A) Truncating mutations (nonsense substitutions and frame-shifting (FS) coding indels) present significantly lower VAF. (B) Change of VAF of mtDNA somatic mutation between primary and metastatic (or late) cancer tissues. (C) Mutational signature for mtDNA across various tumor types. None of the three highlighted mechanisms or nuclear DNA double-strand breaks repair mechanism (BRCA) match with the mtDNA mutational signature. * Only substitutions in protein-coding genes considered. (D) A proposed model of mtDNA mutational process. DOI:
http://dx.doi.org/10.7554/eLife.02935.017
Figure 5—figure supplement 1.. Number of recurrent substitutions between silent and missense substitutions.
100 sites were randomly selected from silent substitutions (at third base of triplet codon) and missense substitutions (at first and second base of triplet codon). No significant difference was observed among these three groups. DOI:
http://dx.doi.org/10.7554/eLife.02935.018
Figure 5—figure supplement 2.. Comparison of VAF of protein-truncating mutations (nonsense substitution and indels) across tumor types.
Four tumor types with more than 10 protein-truncating mutations are shown. Fisher's exact were applied between breast and other tissue types. DOI:
http://dx.doi.org/10.7554/eLife.02935.019
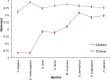
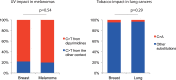
Figure 5—figure supplement 3.. Negligible impacts of external mutagens (UV and tobacco smoking) to the somatic mtDNA mutations.
No evidence of UV and tobacco smoking was identified even in melanoma and lung cancers, respectively. (Left) We compared the proportion of C > T (and G > A) substitutions in the CpC (GpG) context (mutational signature for UV [Alexandrov et al., 2013]) between melanomas and breast cancers (controls). Because UV shows trivial impact to the nuclear DNA somatic mutations of breast cancers (Alexandrov et al., 2013), the vast majority of mtDNA C > T substitutions in the CpC context from breast cancers were not generated by UV. (Right) We compared the proportion of C > A (G > T) substitutions between lung and breast (control) cancers. C > A (G > T) substitutions are dominantly generated by tobacco smoking. Like UV, the impact of tobacco smoking to the somatic mutations of breast cancers is trivial (Alexandrov et al., 2013). DOI:
http://dx.doi.org/10.7554/eLife.02935.020
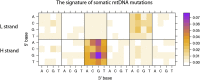
Author response image 1.
Somatic mutational signature of mitochondrial DNA
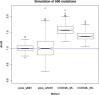
Author response image 2.
dN/dS ratio with 600 somatic substitutions randomly generated
Similar articles
- A replication-linked mutational gradient drives somatic mutation accumulation and influences germline polymorphisms and genome composition in mitochondrial DNA.
Sanchez-Contreras M, Sweetwyne MT, Kohrn BF, Tsantilas KA, Hipp MJ, Schmidt EK, Fredrickson J, Whitson JA, Campbell MD, Rabinovitch PS, Marcinek DJ, Kennedy SR. Sanchez-Contreras M, et al. Nucleic Acids Res. 2021 Nov 8;49(19):11103-11118. doi: 10.1093/nar/gkab901. Nucleic Acids Res. 2021. PMID: 34614167 Free PMC article. - Mitochondrial DNA mutations in pancreatic cancer.
Kassauei K, Habbe N, Mullendore ME, Karikari CA, Maitra A, Feldmann G. Kassauei K, et al. Int J Gastrointest Cancer. 2006;37(2-3):57-64. doi: 10.1007/s12029-007-0008-2. Int J Gastrointest Cancer. 2006. PMID: 17827523 - Mitochondrial DNA mutations in Malaysian female breast cancer patients.
Omasanggar R, Yu CY, Ang GY, Emran NA, Kitan N, Baghawi A, Falparado Ahmad A, Abdullah MA, Teh LK, Maniam S. Omasanggar R, et al. PLoS One. 2020 May 22;15(5):e0233461. doi: 10.1371/journal.pone.0233461. eCollection 2020. PLoS One. 2020. PMID: 32442190 Free PMC article. - Recent Advances in Detecting Mitochondrial DNA Heteroplasmic Variations.
Duan M, Tu J, Lu Z. Duan M, et al. Molecules. 2018 Feb 3;23(2):323. doi: 10.3390/molecules23020323. Molecules. 2018. PMID: 29401641 Free PMC article. Review. - Functional Role of Mitochondrial DNA in Cancer Progression.
Lin YH, Lim SN, Chen CY, Chi HC, Yeh CT, Lin WR. Lin YH, et al. Int J Mol Sci. 2022 Jan 31;23(3):1659. doi: 10.3390/ijms23031659. Int J Mol Sci. 2022. PMID: 35163579 Free PMC article. Review.
Cited by
- Changes in TP53 Gene, Telomere Length, and Mitochondrial DNA in Benign Prostatic Hyperplasia Patients.
Zole E, Baumanis E, Freimane L, Dāle R, Leiše A, Lietuvietis V, Ranka R. Zole E, et al. Biomedicines. 2024 Oct 15;12(10):2349. doi: 10.3390/biomedicines12102349. Biomedicines. 2024. PMID: 39457663 Free PMC article. - Genome-wide quantification of rare somatic mutations in normal human tissues using massively parallel sequencing.
Hoang ML, Kinde I, Tomasetti C, McMahon KW, Rosenquist TA, Grollman AP, Kinzler KW, Vogelstein B, Papadopoulos N. Hoang ML, et al. Proc Natl Acad Sci U S A. 2016 Aug 30;113(35):9846-51. doi: 10.1073/pnas.1607794113. Epub 2016 Aug 15. Proc Natl Acad Sci U S A. 2016. PMID: 27528664 Free PMC article. - Somatic nuclear mitochondrial DNA insertions are prevalent in the human brain and accumulate over time in fibroblasts.
Zhou W, Karan KR, Gu W, Klein HU, Sturm G, De Jager PL, Bennett DA, Hirano M, Picard M, Mills RE. Zhou W, et al. PLoS Biol. 2024 Aug 22;22(8):e3002723. doi: 10.1371/journal.pbio.3002723. eCollection 2024 Aug. PLoS Biol. 2024. PMID: 39172952 Free PMC article. - Mitochondrial DNA is a major source of driver mutations in cancer.
Kim M, Mahmood M, Reznik E, Gammage PA. Kim M, et al. Trends Cancer. 2022 Dec;8(12):1046-1059. doi: 10.1016/j.trecan.2022.08.001. Epub 2022 Aug 27. Trends Cancer. 2022. PMID: 36041967 Free PMC article. Review. - Simultaneous DNA and RNA Mapping of Somatic Mitochondrial Mutations across Diverse Human Cancers.
Stewart JB, Alaei-Mahabadi B, Sabarinathan R, Samuelsson T, Gorodkin J, Gustafsson CM, Larsson E. Stewart JB, et al. PLoS Genet. 2015 Jun 30;11(6):e1005333. doi: 10.1371/journal.pgen.1005333. eCollection 2015 Jun. PLoS Genet. 2015. PMID: 26125550 Free PMC article.
References
- Alexandrov LB, Nik-Zainal S, Wedge DC, Aparicio SA, Behjati S, Biankin AV, Bignell GR, Bolli N, Borg A, Borresen-Dale AL, Boyault S, Burkhardt B, Butler AP, Caldas C, Davies HR, Desmedt C, Eils R, Eyfjörd JE, Foekens JA, Greaves M, Hosoda F, Hutter B, Ilicic T, Imbeaud S, Imielinski M, Jäger N, Jones DT, Jones D, Knappskog S, Kool M, Lakhani SR, López-Otín C, Martin S, Munshi NC, Nakamura H, Northcott PA, Pajic M, Papaemmanuil E, Paradiso A, Pearson JV, Puente XS, Raine K, Ramakrishna M, Richardson AL, Richter J, Rosenstiel P, Schlesner M, Schumacher TN, Span PN, Teague JW, Totoki Y, Tutt AN, Valdés-Mas R, van Buuren MM, van 't Veer L, Vincent-Salomon A, Waddell N, Yates LR, Australian Pancreatic Cancer Genome Initiative , ICGC Breast Cancer Consortium , ICGC MMML-Seq Consortium , ICGC PedBrain. Zucman-Rossi J, Futreal PA, McDermott U, Lichter P, Meyerson M, Grimmond SM, Siebert R, Campo E, Shibata T, Pfister SM, Campbell PJ, Stratton MR. Signatures of mutational processes in human cancer. Nature. 2013;500:415–421. doi: 10.1038/nature12477. - DOI - PMC - PubMed
- Avital G, Buchshtav M, Zhidkov I, Tuval Feder J, Dadon S, Rubin E, Glass D, Spector TD, Mishmar D. Mitochondrial DNA heteroplasmy in diabetes and normal adults: role of acquired and inherited mutational patterns in twins. Human Molecular Genetics. 2012;21:4214–4224. doi: 10.1093/hmg/dds245. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 101876/WT_/Wellcome Trust/United Kingdom
- 17528/CRUK_/Cancer Research UK/United Kingdom
- G0900871/MRC_/Medical Research Council/United Kingdom
- MR/K000608/1/MRC_/Medical Research Council/United Kingdom
- 088340/WT_/Wellcome Trust/United Kingdom
- P01 CA155258/CA/NCI NIH HHS/United States
- 096919/WT_/Wellcome Trust/United Kingdom
- 095663/WT_/Wellcome Trust/United Kingdom
- 15007/CRUK_/Cancer Research UK/United Kingdom
- G1000729/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases