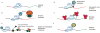Programmable RNA recognition and cleavage by CRISPR/Cas9 - PubMed (original) (raw)
. 2014 Dec 11;516(7530):263-6.
doi: 10.1038/nature13769. Epub 2014 Sep 28.
Affiliations
- PMID: 25274302
- PMCID: PMC4268322
- DOI: 10.1038/nature13769
Programmable RNA recognition and cleavage by CRISPR/Cas9
Mitchell R O'Connell et al. Nature. 2014.
Abstract
The CRISPR-associated protein Cas9 is an RNA-guided DNA endonuclease that uses RNA-DNA complementarity to identify target sites for sequence-specific double-stranded DNA (dsDNA) cleavage. In its native context, Cas9 acts on DNA substrates exclusively because both binding and catalysis require recognition of a short DNA sequence, known as the protospacer adjacent motif (PAM), next to and on the strand opposite the twenty-nucleotide target site in dsDNA. Cas9 has proven to be a versatile tool for genome engineering and gene regulation in a large range of prokaryotic and eukaryotic cell types, and in whole organisms, but it has been thought to be incapable of targeting RNA. Here we show that Cas9 binds with high affinity to single-stranded RNA (ssRNA) targets matching the Cas9-associated guide RNA sequence when the PAM is presented in trans as a separate DNA oligonucleotide. Furthermore, PAM-presenting oligonucleotides (PAMmers) stimulate site-specific endonucleolytic cleavage of ssRNA targets, similar to PAM-mediated stimulation of Cas9-catalysed DNA cleavage. Using specially designed PAMmers, Cas9 can be specifically directed to bind or cut RNA targets while avoiding corresponding DNA sequences, and we demonstrate that this strategy enables the isolation of a specific endogenous messenger RNA from cells. These results reveal a fundamental connection between PAM binding and substrate selection by Cas9, and highlight the utility of Cas9 for programmable transcript recognition without the need for tags.
Figures
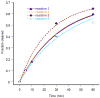
Extended Data Figure 1. Quantified data for cleavage of ssRNA by Cas9–gRNA in the presence of a 19-nt PAMmer
Cleavage assays were conducted as described in the Methods, and the quantified data were fit with single-exponential decays. Results from four independent experiments yielded an average apparent pseudo-first order cleavage rate constant of 0.032 ± 0.007 min-1. This is slower than the rate constant determined previously for ssDNA in the presence of the same 19-nt PAMmer (7.3 ± 3.2 min-1).
Extended Data Figure 2. RNA cleavage is marginally stimulated by di- and trideoxyribonucleotide PAMmers
Cleavage reactions contained ~1 nM 5′-radiolabelled target ssRNA and no PAMmer (left), 100 nM 18-nt PAMmer (second from left), or 1 mM of the indicated di- or tri-nucleotide (remaining lanes). Reaction products were resolved by 12% denaturing polyacrylamide gel electrophoresis (PAGE) and visualized by phosphorimaging.
Extended Data Figure 3. Representative binding experiment demonstrating guide-specific ssRNA binding with 5′-extended PAMmers
Gel shift assays were conducted as described in the Methods. Binding reactions contained Cas9 programmed with λ2 gRNA and either λ2 (on-target), λ3 (off-target) or λ4 (off-target) ssRNA in the presence of short cognate PAMmers or cognate PAMmers with complete 5’-extensions, as indicated. The presence of a cognate 5’-extended PAM-mer abrogates off-target binding. Three independent experiments were conducted to produce the data shown in Fig. 3b, d.
Extended Data Figure 4. Exploration of RNA cleavage efficiencies and binding specificity using PAMmers with variable 5′-extensions
a, Cleavage assays were conducted as described in Methods. Reactions contained Cas9 programmed with λ2 gRNA and λ2 ssRNA target in the presence of PAMmers with 5’-extensions of variable length. The ssRNA cleavage efficiency decreases as the PAMmer extends further into the target region, as indicated by the fraction RNA cleaved after 1 h. b, Binding assays were conducted as described in the Methods, using mostly the same panel of 5’-extended PAMmers as in (a). Binding reactions contained Cas9 programmed with λ2 gRNA and either λ2 (on-target) or λ3 (off-target) ssRNA in the presence of cognate PAMmers with 5’-extensions of variable length. The binding specificity increases as the PAMmer extends further into the target region, as indicated by the fraction of λ3 (off-target) ssRNA bound at 3 nM Cas9-gRNA. PAMmers with 5’ extensions also cause a slight reduction in the relative binding affinity of λ2 (on-target) ssRNA.
Extended Data Figure 5. Site-specific biotin labeling of Cas9
a, In order to introduce a single biotin moiety on Cas9, the solvent accessible, non-conserved N-terminal methionine was mutated to a cysteine (M1C; red text) and the naturally occurring cysteine residues were mutated to serine (C80S and C57S; bold text). This enabled cysteine-specific labeling with EZ-link® Maleimide-PEG2-biotin through an irreversible reaction between the reduced sulfhydryl group of the cysteine and the maleimide group present on the biotin label. dCas9 mutations are also indicated in the domain schematic. b, Mass spectrometry analysis of the Cas9 biotin labeling reaction confirmed that successful biotin labeling only occurs when the M1C mutation is present in the Cys-Free background (C80S, C574S). The mass of the Maleimide-PEG2- biotin reagent is 525.6 Da. c, Streptavidin bead binding assay with biotinylated (biot.) or nonbiotinylated (non-biot.) Cas9 and streptavidin agarose or streptavidin magnetic beads. Cas9 only remains specifically bound to the beads after biotin labeling. d, Cleavage assays were conducted as described in the Methods and resolved by denaturing PAGE. Reactions contained 100 nM Cas9 programmed with λ2 gRNA and ~1 nM 5′-radiolabelled λ2 dsDNA target. e, Quantified cleavage data from triplicate experiments were fit with single-exponential decays to calculate the apparent pseudo-first order cleavage rate constants (average ± standard deviation). Both Cys-Free and Biotin-M1C Cas9 retain WT activity.
Extended Data Figure 6. RNA-guided Cas9 can utilize chemically modified PAMmers
19-nt PAMmer derivatives containing various chemical modifications on the 5’ and 3’ ends (capped) or interspersed still activate Cas9 for cleavage of ssRNA targets. These types of modification are often used to increase the in vivo half-life of short oligonucleotides by preventing exo- and endonuclease-mediated degradation. Cleavage assays were conducted as described in the Methods. PS, phosphorothioate bonds; LNA, locked nucleic acid.
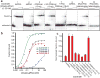
Extended Data Figure 7. Cas9 programmed with GAPDH-specific gRNAs can pull-down GAPDH mRNA in the absence of PAMmer
a, Northern blot showing that, in some cases, Cas9-gRNA is able to pull down detectable amounts of GAPDH mRNA from total RNA without requiring a PAMmer. a, Northern blot showing that Cas9-gRNA 1 is also able to pull-down quantitative amounts of GAPDH mRNA from HeLa cell lysate without requiring a PAMmer. s: standard; v: 2’-OMe-modified PAMmers.
Extended Data Figure 8. Potential applications of RCas9 for untagged transcript analysis, detection, and manipulation
a, Catalytically-active RCas9 could be used to target and cleave RNA, particularly those for which RNAi-mediated repression/degradation is not possible. b, Tethering the eukaryotic initiation factor eIF4G to a catalytically inactive dRCas9 targeted to the 5’ untranslated region of an mRNA could drive translation. c, dRCas9 tethered to beads could be used to specificially isolate RNA or native RNA:protein complexes of interest from cells for downstream analysis or assays including identification of bound protein complexes, probing of RNA structure under native protein-bound conditions, and enrichment of rare transcripts for sequencing analysis. d, dRCas9 tethered to RNA deaminase or N-mA methylase domains could direct site-specific A-to-I editing or methylation or RNA, respectively. e, dRCas9 fused to a U1 recruitment domain (arginine- and serine-rich (RS) domain) could be programmed to recognize a splicing enhancer site and thereby promote the inclusion of a targeted exon. f, dRCas9 tethered to a fluorescent protein such as GFP could be used to observe RNA localization and transport in living cells.
Figure 1. RNA-guided Cas9 cleaves ssRNA targets in the presence of a short PAM-presenting DNA oligonucleotide (PAMmer)
a, Schematic depicting the approach used to target ssRNA for programmable, sequence-specific cleavage. b, The panel of nucleic acid substrates examined in this study. Substrate elements are colored as follows: DNA (grey), RNA (black), guide RNA target sequence (red), DNA PAM (yellow), mutated DNA PAM (blue), RNA PAM (orange). c, Representative cleavage assay for 5’-radiolabeled nucleic acid substrates using Cas9–gRNA, numbered as in (b). d, Cas9–gRNA cleavage site mapping assay for substrate 3. T1 and OH- denote RNase T1 and hydrolysis ladders, respectively; the sequence of the target ssRNA is shown at right. e, Representative ssRNA cleavage assay in the presence of PAMmers of increasing length, numbered as in (b).
Figure 2. dCas9–gRNA binds ssRNA targets with high affinity in the presence of PAMmers
a, Representative electrophoretic mobility shift assay for binding reactions with dCas9–gRNA and a panel of 5′-radiolabeled nucleic acid substrates, numbered as in Fig. 1b. b, Quantified binding data for substrates 1–4 from (a) fit with standard binding isotherms. Measured dissociation constants from three independent experiments (mean ± s.d.) were 0.036 ± 0.003 nM (1), >100 nM (2), 0.20 ± 0.09 nM (3), and 0.18 ± 0.07 nM (4). c, Relative binding data for 1 nM dCas9–gRNA and 5’-radiolabeled ssRNA with a panel of different PAMmers. The data are normalized to the amount of binding observed at 1 nM dCas9–gRNA with a 19-nt PAMmer; error bars represent the standard deviation from three independent experiments.
Figure 3. 5’-extended PAMmers are required for specific target ssRNA binding
a, Cas9 programmed with either λ2-, λ3-, or λ4-targeting gRNAs exhibits sequence-specific cleavage of 5’-radiolabeled λ2, λ3, and λ4 target ssRNAs, respectively, in the presence of cognate PAMmers. b, dCas9 programmed with a λ2-targeting gRNA exhibits similar binding affinity to λ2, λ3, and λ4 target ssRNAs in the presence of cognate PAMmers. Dissociation constants from three independent experiments (mean ± s.d.) were 0.20 ± 0.09 nM (λ2), 0.33 ± 0.14 nM (λ3), and 0.53 ± 0.21 nM (λ4). c, Schematic depicting the approach used to restore guide RNA-mediated ssRNA binding specificity, which involves 5’-extensions to the PAMmer that cover part of the target sequence. d, dCas9 programmed with a λ2-targeting gRNA specifically binds the λ2 ssRNA but not λ3 and λ4 ssRNAs in the presence of 5’-extended PAMmers. Dissociation constants from three independent experiments (mean ± s.d.) were 3.3 ± 1.2 nM (λ2) and >100 nM (λ3 and λ4).
Figure 4. RNA-guided Cas9 can target non-PAM sites on ssRNA and isolate GAPDH mRNA from HeLa cells in a tagless manner
a,Schematic of the approach designed to avoid cleavage of template DNA by targeting non-PAM sites in the ssRNA target. b, The panel of nucleic acid substrates tested in (c). c, Cas9–gRNA cleaves ssRNA targets with equal efficiency when the 5’-NGG-3’ of the PAMmer is mismatched with the ssRNA. This strategy enables selective cleavage of ssRNA in the presence of non-PAM target dsDNA. d, Schematic of the dCas9 RNA pull-down experiment. e, GAPDH mRNA transcript isoform 3 shown schematically, with exons common to all GAPDH protein-coding transcripts in red and gRNA/PAMmer targets _G_1-_G_4 indicated. f, Northern blot showing that gRNAs and 5’-extended PAMmers enable tagless isolation of GAPDH mRNA from HeLa total RNA; β-actin mRNA is shown as a control. g, Northern blot showing tagless isolation of GAPDH mRNA from HeLa cell lysate with varying 2’-OMe-modified PAMmers. RNase H cleavage is abrogated with v4 and v5 PAMmers; β-actin mRNA is shown as a control. h, Sequences of unmodified and modified GAPDH PAMmers used in (g); 2’-OMe-modified nucleotides are shown in red.
Comment in
- Genetics: Cleaving RNA with Cas9.
Ghodsizadeh O. Ghodsizadeh O. Nat Methods. 2014 Nov;11(11):1090. doi: 10.1038/nmeth.3164. Nat Methods. 2014. PMID: 25551124 No abstract available.
Similar articles
- DNA interrogation by the CRISPR RNA-guided endonuclease Cas9.
Sternberg SH, Redding S, Jinek M, Greene EC, Doudna JA. Sternberg SH, et al. Nature. 2014 Mar 6;507(7490):62-7. doi: 10.1038/nature13011. Epub 2014 Jan 29. Nature. 2014. PMID: 24476820 Free PMC article. - CRISPR-Cas9 Structures and Mechanisms.
Jiang F, Doudna JA. Jiang F, et al. Annu Rev Biophys. 2017 May 22;46:505-529. doi: 10.1146/annurev-biophys-062215-010822. Epub 2017 Mar 30. Annu Rev Biophys. 2017. PMID: 28375731 Review. - The CRISPR-associated DNA-cleaving enzyme Cpf1 also processes precursor CRISPR RNA.
Fonfara I, Richter H, Bratovič M, Le Rhun A, Charpentier E. Fonfara I, et al. Nature. 2016 Apr 28;532(7600):517-21. doi: 10.1038/nature17945. Epub 2016 Apr 20. Nature. 2016. PMID: 27096362 - RNA-dependent RNA targeting by CRISPR-Cas9.
Strutt SC, Torrez RM, Kaya E, Negrete OA, Doudna JA. Strutt SC, et al. Elife. 2018 Jan 5;7:e32724. doi: 10.7554/eLife.32724. Elife. 2018. PMID: 29303478 Free PMC article. - Expanding the Biologist's Toolkit with CRISPR-Cas9.
Sternberg SH, Doudna JA. Sternberg SH, et al. Mol Cell. 2015 May 21;58(4):568-74. doi: 10.1016/j.molcel.2015.02.032. Mol Cell. 2015. PMID: 26000842 Review.
Cited by
- Integration of CRISPR/Cas9 with multi-omics technologies to engineer secondary metabolite productions in medicinal plant: Challenges and Prospects.
Borah A, Singh S, Chattopadhyay R, Kaur J, Bari VK. Borah A, et al. Funct Integr Genomics. 2024 Nov 4;24(6):207. doi: 10.1007/s10142-024-01486-w. Funct Integr Genomics. 2024. PMID: 39496976 Review. - Synergistic effect of split DNA activators of Cas12a with exon-unwinding and induced targeting effect.
Huang S, Lou Y, Zheng L. Huang S, et al. Nucleic Acids Res. 2024 Oct 14;52(18):11148-11157. doi: 10.1093/nar/gkae766. Nucleic Acids Res. 2024. PMID: 39258555 Free PMC article. - Role of CRISPR-Cas systems and anti-CRISPR proteins in bacterial antibiotic resistance.
Kadkhoda H, Gholizadeh P, Samadi Kafil H, Ghotaslou R, Pirzadeh T, Ahangarzadeh Rezaee M, Nabizadeh E, Feizi H, Aghazadeh M. Kadkhoda H, et al. Heliyon. 2024 Jul 16;10(14):e34692. doi: 10.1016/j.heliyon.2024.e34692. eCollection 2024 Jul 30. Heliyon. 2024. PMID: 39149034 Free PMC article. Review. - Hypoimmunogenic human iPSCs expressing HLA-G, PD-L1, and PD-L2 evade innate and adaptive immunity.
Tsuneyoshi N, Hosoya T, Takeno Y, Saitoh K, Murai H, Amimoto N, Tatsumi R, Watanabe S, Hasegawa Y, Kikkawa E, Goto K, Nishigaki F, Tamura K, Kimura H. Tsuneyoshi N, et al. Stem Cell Res Ther. 2024 Jul 2;15(1):193. doi: 10.1186/s13287-024-03810-4. Stem Cell Res Ther. 2024. PMID: 38956724 Free PMC article. - Nanoliposomes as nonviral vectors in cancer gene therapy.
Yildiz SN, Entezari M, Paskeh MDA, Mirzaei S, Kalbasi A, Zabolian A, Hashemi F, Hushmandi K, Hashemi M, Raei M, Goharrizi MASB, Aref AR, Zarrabi A, Ren J, Orive G, Rabiee N, Ertas YN. Yildiz SN, et al. MedComm (2020). 2024 Jun 25;5(7):e583. doi: 10.1002/mco2.583. eCollection 2024 Jul. MedComm (2020). 2024. PMID: 38919334 Free PMC article. Review.
References
- Wiedenheft B, Sternberg SH, Doudna JA. RNA-guided genetic silencing systems in bacteria and archaea. Nature. 2012;482:331–338. - PubMed
- Barrangou R, et al. CRISPR provides acquired resistance against viruses in prokaryotes. Science. 2007;315:1709–1712. - PubMed
- Garneau JE, et al. The CRISPR/Cas bacterial immune system cleaves bacteriophage and plasmid DNA. Nature. 2010;468:67–72. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32 GM007232/GM/NIGMS NIH HHS/United States
- T32 GM066698/GM/NIGMS NIH HHS/United States
- P50 GM102706/GM/NIGMS NIH HHS/United States
- P50GM102706-03/GM/NIGMS NIH HHS/United States
- HHMI_/Howard Hughes Medical Institute/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous