Single-step isolation of extracellular vesicles by size-exclusion chromatography - PubMed (original) (raw)
Single-step isolation of extracellular vesicles by size-exclusion chromatography
Anita N Böing et al. J Extracell Vesicles. 2014.
Abstract
Background: Isolation of extracellular vesicles from plasma is a challenge due to the presence of proteins and lipoproteins. Isolation of vesicles using differential centrifugation or density-gradient ultracentrifugation results in co-isolation of contaminants such as protein aggregates and incomplete separation of vesicles from lipoproteins, respectively.
Aim: To develop a single-step protocol to isolate vesicles from human body fluids.
Methods: Platelet-free supernatant, derived from platelet concentrates, was loaded on a sepharose CL-2B column to perform size-exclusion chromatography (SEC; n=3). Fractions were collected and analysed by nanoparticle tracking analysis, resistive pulse sensing, flow cytometry and transmission electron microscopy. The concentrations of high-density lipoprotein cholesterol (HDL) and protein were measured in each fraction.
Results: Fractions 9-12 contained the highest concentrations of particles larger than 70 nm and platelet-derived vesicles (46%±6 and 61%±2 of totals present in all collected fractions, respectively), but less than 5% of HDL and less than 1% of protein (4.8%±1 and 0.65%±0.3, respectively). HDL was present mainly in fractions 18-20 (32%±2 of total), and protein in fractions 19-21 (36%±2 of total). Compared to the starting material, recovery of platelet-derived vesicles was 43%±23 in fractions 9-12, with an 8-fold and 70-fold enrichment compared to HDL and protein.
Conclusions: SEC efficiently isolates extracellular vesicles with a diameter larger than 70 nm from platelet-free supernatant of platelet concentrates. Application SEC will improve studies on the dimensional, structural and functional properties of extracellular vesicles.
Keywords: extracellular vesicles; isolation; lipoproteins; plasma; protein; size-exclusion chromatography.
Figures
Fig. 1
Image of size-exclusion chromatography column. A 10 mL syringe stacked with sepharose CL-2B for isolation of vesicles from platelet-free supernatant of platelet concentrates.
Fig. 2
Presence of vesicles, protein and lipoproteins per fraction. The concentration of vesicles, protein and lipoproteins was measured in each fraction. Each bar shows the number present in a fraction as % of the total number that passed the column. The height of the bar represents the mean, the error bars the standard deviation from 3 experiments. a) Particles (larger than 70 nm) measured by NTA. b) Particles (100–400 nm) measured by RPS. c) CD61+ vesicles measured by flow cytometry. d) Lactadherin+vesicles measured by flow cytometry. e) HDL (Cholesterol) concentration measured by a colorimetric assay. f) HDL (APO A1) concentration measured by a turbidimetric assay. g) Protein concentration measured by a Bradford protein assay h) Overview of all measured results.
Fig. 3
Presence of proteins, CD63 and CD9 in collected fractions. a) The presence of proteins in each fraction determined by loading 20 µL on PAGE gels. The molecular weight of albumin is 66 kDa. b,c) Presence of tetraspanins in the different fractions was studied by Western blot, with 4 µg protein used per fraction. First, the presence of CD63 was shown (53 kDa, panel b), and next the presence of CD9 was shown (24 kDa, panel c). The tetraspanin bands are indicated by arrows in panels b and c. Platelet lysate was used as positive control.
Fig. 4
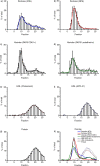
TEM images of fractions. Starting material and fractions, undiluted or 50-fold diluted when indicated, were analysed by TEM for the presence of particles. All images shown are representative images for the starting material (a, j) and fractions 5, 9, 10, 11, 17, 18, 19 and 20 (b–i, k–n). Scale bar is 200 nm (a–g, j–n), 500 nm (h), or 1 µm (i).
Fig. 5
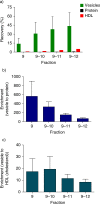
Recovery and enrichment. The recovery and enrichment relative to the starting material, in the vesicle-containing fractions (9, 9–10, 9–11, 9–12) are shown. a) Recovery of vesicles, protein and HDL (cholesterol) in the vesicle-containing fractions. b) Enrichment factor of vesicle to protein. c) Enrichment factor of vesicle to HDL (cholesterol).
Similar articles
- Isolation of High-Purity Extracellular Vesicles by the Combination of Iodixanol Density Gradient Ultracentrifugation and Bind-Elute Chromatography From Blood Plasma.
Onódi Z, Pelyhe C, Terézia Nagy C, Brenner GB, Almási L, Kittel Á, Manček-Keber M, Ferdinandy P, Buzás EI, Giricz Z. Onódi Z, et al. Front Physiol. 2018 Oct 23;9:1479. doi: 10.3389/fphys.2018.01479. eCollection 2018. Front Physiol. 2018. PMID: 30405435 Free PMC article. - Extracellular Vesicles from Human Cerebrospinal Fluid Are Effectively Separated by Sepharose CL-6B-Comparison of Four Gravity-Flow Size Exclusion Chromatography Methods.
Krušić Alić V, Malenica M, Biberić M, Zrna S, Valenčić L, Šuput A, Kalagac Fabris L, Wechtersbach K, Kojc N, Kurtjak M, Kučić N, Grabušić K. Krušić Alić V, et al. Biomedicines. 2022 Mar 27;10(4):785. doi: 10.3390/biomedicines10040785. Biomedicines. 2022. PMID: 35453535 Free PMC article. - Ultrafiltration combined with size exclusion chromatography efficiently isolates extracellular vesicles from cell culture media for compositional and functional studies.
Benedikter BJ, Bouwman FG, Vajen T, Heinzmann ACA, Grauls G, Mariman EC, Wouters EFM, Savelkoul PH, Lopez-Iglesias C, Koenen RR, Rohde GGU, Stassen FRM. Benedikter BJ, et al. Sci Rep. 2017 Nov 10;7(1):15297. doi: 10.1038/s41598-017-15717-7. Sci Rep. 2017. PMID: 29127410 Free PMC article. - Human lymphedema fluid lipoproteins: particle size, cholesterol and apolipoprotein distributions, and electron microscopic structure.
Reichl D, Forte TM, Hong JL, Rudra DN, Pflug J. Reichl D, et al. J Lipid Res. 1985 Dec;26(12):1399-411. J Lipid Res. 1985. PMID: 4086943 - Isolation of Cell-Free miRNA from Biological Fluids: Influencing Factors and Methods.
Bryzgunova O, Konoshenko M, Zaporozhchenko I, Yakovlev A, Laktionov P. Bryzgunova O, et al. Diagnostics (Basel). 2021 May 11;11(5):865. doi: 10.3390/diagnostics11050865. Diagnostics (Basel). 2021. PMID: 34064927 Free PMC article. Review.
Cited by
- Separation of small extracellular vesicles (sEV) from human blood by Superose 6 size exclusion chromatography.
Nouvel J, Bustos-Quevedo G, Prinz T, Masood R, Daaboul G, Gainey-Schleicher T, Wittel U, Chikhladze S, Melykuti B, Helmstaedter M, Winkler K, Nazarenko I, Pütz G. Nouvel J, et al. J Extracell Vesicles. 2024 Oct;13(10):e70008. doi: 10.1002/jev2.70008. J Extracell Vesicles. 2024. PMID: 39441012 Free PMC article. - Rapid separation of blood plasma exosomes from low-density lipoproteins via a hydrophobic interaction chromatography method on a polyester capillary-channeled polymer fiber phase.
Huang S, Ji X, Jackson KK, Lubman DM, Ard MB, Bruce TF, Marcus RK. Huang S, et al. Anal Chim Acta. 2021 Jul 4;1167:338578. doi: 10.1016/j.aca.2021.338578. Epub 2021 Apr 29. Anal Chim Acta. 2021. PMID: 34049630 Free PMC article. - Extracellular vesicles biogenesis, isolation, manipulation and genetic engineering for potential in vitro and in vivo therapeutics: An overview.
Hadizadeh N, Bagheri D, Shamsara M, Hamblin MR, Farmany A, Xu M, Liang Z, Razi F, Hashemi E. Hadizadeh N, et al. Front Bioeng Biotechnol. 2022 Nov 4;10:1019821. doi: 10.3389/fbioe.2022.1019821. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 36406206 Free PMC article. Review. - Extracellular Vesicle Mobility in Collagen I Hydrogels Is Influenced by Matrix-Binding Integrins.
Tam NW, Becker A, Mangiarotti A, Cipitria A, Dimova R. Tam NW, et al. ACS Nano. 2024 Oct 29;18(43):29585-29601. doi: 10.1021/acsnano.4c07186. Epub 2024 Oct 14. ACS Nano. 2024. PMID: 39400273 Free PMC article. - Extracellular Vesicles: Novel Roles in Neurological Disorders.
Jin Q, Wu P, Zhou X, Qian H, Xu W. Jin Q, et al. Stem Cells Int. 2021 Feb 17;2021:6640836. doi: 10.1155/2021/6640836. eCollection 2021. Stem Cells Int. 2021. PMID: 33679989 Free PMC article. Review.
References
- Tesselaar ME, Romijn FP, Van Der Linden IK, Prins FA, Bertina RM, Osanto S. Microparticle-associated tissue factor activity: a link between cancer and thrombosis? J Thromb Haemost. 2007;5:520–7. - PubMed
- van Doormaal F, Kleinjan A, Berckmans RJ, Mackman N, Manly D, Kamphuisen PW, et al. Coagulation activation and microparticle-associated coagulant activity in cancer patients. An exploratory prospective study. Thromb Haemost. 2012;108:160–5. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources