Extracellular recordings reveal absence of magneto sensitive units in the avian optic tectum - PubMed (original) (raw)
Extracellular recordings reveal absence of magneto sensitive units in the avian optic tectum
Edgardo Ramírez et al. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2014 Dec.
Abstract
There is a consensus that birds detect the earth's magnetic field and use some of its features for orientation and homing purposes. Since the late 1960s, when the first solid behavioral evidence of magnetoreception was obtained, much research has been devoted to describing the ethological aspects of this behavior. The neurophysiological basis of magnetoreception has been much less studied, although a frequently cited 1986 report described a high prevalence (70 %) of magneto-sensitive neurons in the pigeon optic tectum with high signal-to-noise ratios (Semm and Demaine, J Comp Physiol A 159:619-625, 1986). Here, we repeated these neurophysiological experiments using anesthetized as well as awake pigeons and new recording techniques. Our data indicate that magneto-sensitive units do not exist in the avian tectum.
Figures
Fig. 1
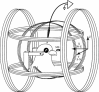
The stimulation setup consisted of three orthogonal Helmholtz coils. The total magnitude of the artificial field was kept similar to the amplitude found in Santiago. Most of the experiments recorded from the left tectum and the right eye was illuminated with LEDs located at 50 cm behind a translucent screen. A 3D magnetic sensor (MS) was located near the pigeon beak. The coils and LEDs were powered by high current 12 V DC batteries. In experiments with awake pigeons the ear bars were not used. The coils left most of the right visual field unobstructed
Fig. 2
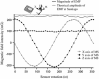
Properties of the 3D magnetic sensor (MS) under a rotation around the Z axis. The sensor was placed horizontally and rotated manually around the Z axis in 10° steps while recording its three outputs. As expected, the X (asterisk) and Y (filled circle) outputs were in quadrature while the Z (filled triangle) output was almost constant. The total magnitude of the recorded field was 5 % lower (gray line) than the theoretical value of the earth’s magnetic field (EMF) at Santiago according to the NOAA-WMM 2010 model (dotted line)
Fig. 3
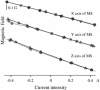
Behavior of Helmholtz coils. The magnetic intensity produced by each Helmholtz coil pair used in isolation (open triangle) is a linear function of the current traversing each pair (data for all three pairs). When the three coils are energized concurrently, the deviations are minor (filled circle). Thus, the X, Y and Z components of the induced magnetic field are almost orthogonal
Fig. 4
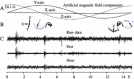
Neural data under magnetic stimulation in an awake pigeon under blue light. The applied magnetic field starts vertically aligned with the Z axis, and it is rotated, counterclockwise, in the (Z, Y) plane in 15 s. a The X, Y, Z outputs of the magnetic sensor show the expected sinusoidal variations around the Z and Y axes, b diagram showing the relative position of the magnetic field reference frame with respect to the pigeon’s head, the small sphere shows the movement of the magnetic vector with respect to the pigeon’s head (left inset = scan begin; right insert = scan end). c Neural signals (raw, fast and slow) obtained during magnetic stimulation. Bursty activity occurs at 1, 4.5 and 13.5 s, but it is not related to the applied magnetic field
Fig. 5
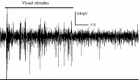
Neural data under visual stimulation in awake pigeon. Tectal activity (raw signal) elicited by the slow movement of a 1° spot traversing a tectal receptive field. The stimulus was manually moved during the first 25 s and trigger strong and clear responses. When the movement ceased, neural activity disappeared. This response shows how strongly tectal units respond to visual motion
Fig. 6
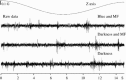
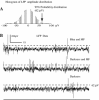
Neural data under different stimulation conditions in awake pigeon. Raw activity traces obtained under three experimental conditions; blue illumination with artificial magnetic field (upper trace), darkness with artificial magnetic field (middle trace) and darkness with no artificial magnetic field (lower trace). In all three conditions, tectal bursts are visible
Fig. 7
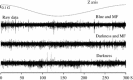
Neural data under magnetic stimulation in an awake pigeon under blue light, at 1.2°/s. Tectal raw activity elicited by the slow scan (300 s) of the artificial magnetic field under three stimulation conditions. No modulation of neural activity by the magnetic field was apparent (compare with Fig. 6)
Fig. 8
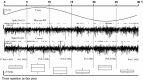
Segment method to assess responsivity of tectal units in a magnetic field (spike signal). The two neural signals represent the multiunit activity of tectal neurons in control (third trace) and in the presence of a magnetic field and blue light (second trace) for the indicated magnetic field (first trace)—above neural traces spikes are represented by dots. A first observation reveals that the raw activity (measured in terms of total number of detected spikes per stimulation cycle) is not different between the two conditions (153 vs 162 spikes). As these experiments were done in an awake pigeons, a spontaneous and variable discharge was observed. To assess a possible magnetic effect, the complete run was divided in six 5 s segments. The total number of spikes in each segment in both conditions was compared with the U Mann–Whitney test (aggregating the three repetitions done for every condition). The _U_-statistics, given below the control trace, show that no difference could be detected for experimental and control conditions [the _p_-statistics were larger that 0.01 thus differences were not significant (NS)]. Furthermore, to show the intrinsic variability of discharge rate, we considered together the spike data from control and experimental conditions, and we represented these sets (6 numbers) by their boxplots (lower panel). It is immediately apparent how variable is the discharge rate in awake pigeons (compare boxplots from the second and fourth segments). In all nine experiments we could not detect a modulation of the discharge rate
Fig. 9
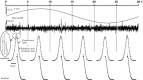
Method to assess responsivity of tectal units to magnetic field using LFP amplitudes. The slow signal from the control situation was used to build a histogram of amplitudes (a). The _x_-coordinate corresponding to 99 % was calculated (arrow). This value was used to calculate the amount of time that neural signals in experimental conditions were above this value. If the amount of time that the signal was above this limit was higher than 2 % we counted it as a positive event (b)
Fig. 10
Segment method to assess responsivity of tectal units to magnetic field (LFP signal). A tectal unit was stimulated by varying the vertical component of the magnetic field in 30 s for a complete 360° excursion (first row). To assess the existence of possible magnetic responses the 30 s segment was subdivided in six 5-s segments. For each segment we calculate, for control (no magnetic field, trace not shown) and stimulated conditions (magnetic field + blue light, second row), the histogram of amplitude values (third row) or the histogram for rectified amplitudes (fourth row) of the LFP signal. No differences were detected for direct or rectified amplitudes. The empirical distributions were similar for control (dot) or for stimulated (continuous curve) conditions (third and fourth rows). The small inset, third row, shows the standard deviations in both cases, and when they are taken into account, no significant differences are detected between control and experimental conditions. Thus, the magnetic field does not stimulate tectal neurons as it does not influence the amplitude of LFP signals (either direct or rectified) in specific phases of the magnetic field
Fig. 11
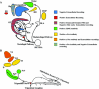
Summary of neurophysiology of avian magnetoreception research. The search for the neural basis of magnetoreception used two different tools: extracellular recordings and activity markers (_c_-fos, ZENK). Positive electrophysiological results have been reported in: the pineal gland (PG) (Semm 1983), the trigeminal ganglion (Semm and Beason 1990a, b), the superior (VS) and lateral (VeL) vestibular nuclei (Wu and Dickman 2012), the nucleus of the basal optic root (nBOR) (Semm and Demaine 1986) and the optic tectum (Semm and Demaine 1986). Negative electrophysiological results have been reported in the optic tectum, the nucleus isthmi pars parvocellularis (Ipc), the entopallium and the hippocampus (Hp) (data from Rose and Ramirez 2011). Positive evidence derived from activity markers has been found in the Wulst, and subdivisions of the hyperpallium (HD, DMP) (Mouritsen et al. ; Heyers et al. , ; Mouritsen and Hore ; Mouritsen 2013). Also subdivisions of the trigeminal nucleus labeling (PrV: Principal sensory nucleus of the trigeminal nerve, SpV: Spinal trigeminal nucleus) (data from Heyers et al. ; Wu and Dickman 2011). Some important nuclei, because of their physiology and position in the visual pathways, have not been explored: the nucleus rotundus (Rt), the nucleus of the dorsal thalamus (DLA) and the isthmo optic nucleus (IOn)
Similar articles
- Synchronization of neuronal responses in the optic tectum of awake pigeons.
Neuenschwander S, Engel AK, König P, Singer W, Varela FJ. Neuenschwander S, et al. Vis Neurosci. 1996 May-Jun;13(3):575-84. doi: 10.1017/s0952523800008257. Vis Neurosci. 1996. PMID: 8782385 - Processing of motion stimuli by cells in the optic tectum of chickens.
Verhaal J, Luksch H. Verhaal J, et al. Neuroreport. 2015 Jul 8;26(10):578-82. doi: 10.1097/WNR.0000000000000391. Neuroreport. 2015. PMID: 26053699 - Locialization of directionally selective and movement sensitive cells in the optic tectum of the pigeon.
Jassik-Gerschenfeld D, Guichard J, Tessier Y. Jassik-Gerschenfeld D, et al. Vision Res. 1975 Aug-Sep;15:1037-8. doi: 10.1016/0042-6989(75)90250-3. Vision Res. 1975. PMID: 1166602 No abstract available. - Magnetic compass orientation in birds and its physiological basis.
Wiltschko W, Wiltschko R. Wiltschko W, et al. Naturwissenschaften. 2002 Oct;89(10):445-52. doi: 10.1007/s00114-002-0356-5. Epub 2002 Sep 13. Naturwissenschaften. 2002. PMID: 12384718 Review. - Neurobiology of the homing pigeon--a review.
Mehlhorn J, Rehkämper G. Mehlhorn J, et al. Naturwissenschaften. 2009 Sep;96(9):1011-25. doi: 10.1007/s00114-009-0560-7. Epub 2009 Jun 2. Naturwissenschaften. 2009. PMID: 19488733 Review.
Cited by
- Electrophysiology and the magnetic sense: a guide to best practice.
Fenton GE, Nath K, Malkemper EP. Fenton GE, et al. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2022 Jan;208(1):185-195. doi: 10.1007/s00359-021-01517-y. Epub 2021 Oct 29. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2022. PMID: 34713390 Free PMC article. Review. - Radical-pair-based magnetoreception in birds: radio-frequency experiments and the role of cryptochrome.
Nießner C, Winklhofer M. Nießner C, et al. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2017 Jul;203(6-7):499-507. doi: 10.1007/s00359-017-1189-1. Epub 2017 Jun 13. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2017. PMID: 28612234 Free PMC article. - Integration and evaluation of magnetic stimulation in physiology setups.
Ahlers MT, Block CT, Winklhofer M, Greschner M. Ahlers MT, et al. PLoS One. 2022 Jul 22;17(7):e0271765. doi: 10.1371/journal.pone.0271765. eCollection 2022. PLoS One. 2022. PMID: 35867646 Free PMC article. - Magnetoreception in birds.
Wiltschko R, Wiltschko W. Wiltschko R, et al. J R Soc Interface. 2019 Sep 27;16(158):20190295. doi: 10.1098/rsif.2019.0295. Epub 2019 Sep 4. J R Soc Interface. 2019. PMID: 31480921 Free PMC article. Review. - Rate and success of study replication in ecology and evolution.
Kelly CD. Kelly CD. PeerJ. 2019 Sep 10;7:e7654. doi: 10.7717/peerj.7654. eCollection 2019. PeerJ. 2019. PMID: 31565572 Free PMC article.
References
- Able KP. Magnetic orientation and magnetoreception in birds. Prog Neurobiol. 1994;42:449–473. - PubMed
- Ahmad M, Galland P, Ritz T, Wiltschko R, Wiltschko W. Magnetic intensity affects cryptochrome-dependent responses in Arabidopsis thaliana. Planta. 2007;225(3):615–624. - PubMed
- Azanza MJ, Del Moral A. Cell-membrane biochemistry and neurobiological approach to biomagnetism. Prog Neurobiol. 1994;44(6):517–601. - PubMed
- Beason RC. Mechanisms of magnetic orientation in birds. Integr Comp Biol. 2005;45:565–573. - PubMed
- Beason RC, Dussourd N, Deutschlander ME. Behavioral evidence for the use of magnetic material in magnetoreception by migratory bird. J Exp Biol. 1995;198:141–146. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous