Reduced sensitivity to both positive and negative reinforcement in mice over-expressing the 5-hydroxytryptamine transporter - PubMed (original) (raw)
. 2014 Dec;40(12):3735-45.
doi: 10.1111/ejn.12744. Epub 2014 Oct 4.
Affiliations
- PMID: 25283165
- PMCID: PMC4737229
- DOI: 10.1111/ejn.12744
Reduced sensitivity to both positive and negative reinforcement in mice over-expressing the 5-hydroxytryptamine transporter
Samantha J Line et al. Eur J Neurosci. 2014 Dec.
Abstract
The 5-hydroxytryptamine (5-HT) transporter (5-HTT) is believed to play a key role in both normal and pathological psychological states. Much previous data suggest that the s allele of the polymorphic regulatory region of the 5-HTT gene promoter is associated with reduced 5-HTT expression and vulnerability to psychiatric disorders, including anxiety and depression. In comparison, the l allele, which increases 5-HTT expression, is generally considered protective. However, recent data link this allele to both abnormal 5-HT signalling and psychopathic traits. Here, we studied the processing of aversive and rewarding cues in transgenic mice that over-express the 5-HTT (5-HTTOE mice). Compared with wild-type mice, 5-HTTOE mice froze less in response to both a tone that had previously been paired with footshock, and the conditioning context. In addition, on a decision-making T-maze task, 5-HTTOE mice displayed reduced preference for a larger, delayed reward and increased preference for a smaller, immediate reward, suggesting increased impulsiveness compared with wild-type mice. However, further inspection of the data revealed that 5-HTTOE mice displayed a relative insensitivity to reward magnitude, irrespective of delay. In contrast, 5-HTTOE mice appeared normal on tests of spatial working and reference memory, which required an absolute choice between options associated with either reward or no reward. Overall, the present findings suggest that 5-HTT over-expression results in a reduced sensitivity to both positive and negative reinforcers. Thus, these data show that increased 5-HTT expression has some maladaptive effects, supporting recent suggestions that l allele homozygosity may be a potential risk factor for disabling psychiatric traits.
Keywords: decision-making; fear conditioning; serotonin; spatial memory.
© 2014 The Authors. European Journal of Neuroscience published by Federation of European Neuroscience Societies and John Wiley & Sons Ltd.
Figures
Figure 1
Apparatus for cost/benefit decision‐making experiment. (A) T‐maze seen from above. One goal arm always contained a low reward (
LR
) and one arm always contained a high reward (
HR
). Guillotine doors were located at the entrances of the goal arms (A) and in front of the food wells (B). (B) Lateral view of the goal arm to demonstrate movement of the doors for introduction of a delay.
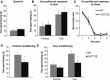
Figure 2
5‐
HT
transporter (5‐
HTT
) over‐expressing (5‐
HTTOE
) mice display impaired fear conditioning. (A) Baseline freezing during training. A 6‐min acclimatisation period occurred before the first tone–shock presentation. Mean percent time freezing (+
SEM
) during this period. (B) Unconditioned response to the first tone during training. Freezing during the 4 s prior to the first tone was compared with freezing during the 4 s immediately after tone onset. Mean percent time freezing (+
SEM
). (C) Unconditioned response to the first shock. The amount of rapid (‘burst’) activity was recorded for 4 s from the onset of the 0.5‐s footshock. Mean burst activity in seconds (±
SEM
). (D) Context fear conditioning. Graph shows the mean percent time spent freezing (+
SEM
) when animals were returned to the training context 24 h later. (E) Cue fear conditioning. The 30‐s cue was re‐presented twice in a novel context and the mean percent time spent freezing (+
SEM
) for time bins prior (pre‐tone) and during (tone) the tone are shown. Wild‐type mice (light bars/light solid line), n = 10; 5‐
HTTOE
mice (dark bars/dark dashed line), n = 10. *P < 0.05 compared with wild‐types.
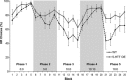
Figure 3
Impulsive choice on a T‐maze cost/benefit decision‐making task in 5‐
HT
transporter (5‐
HTT
) over‐expressing (5‐
HTTOE
) mice. Cost/benefit decision‐making experiment. Values represent the mean percent of high reward (
HR
) arm choices per block of 10 trials ±
SEM
. For Phases 1–5, the ratios for each phase depicted along the bottom of the figure represent ‘the number of seconds delay in the
HR
arm; number of seconds delay in the
LR
arm’. Twenty forced trials (10 to the
HR
arm and 10 to the
LR
arm) were given between the end of Phase 4 and the start of Phase 5. Wild‐types (light solid line; n = 10), 5‐
HTTOE
mice (dark dashed line; n = 11).
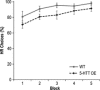
Figure 4
Altered reward discrimination performance in 5‐
HT
transporter (5‐
HTT
) over‐expressing (5‐
HTTOE
) mice. Mean percent high reward (
HR
) arm choices ±
SEM
during Phase 1 of the decision‐making experiment (acquisition of the reward magnitude discrimination), showing data for all animals that completed this phase. Wild‐types (light solid line; n = 11), 5‐
HTTOE
mice (dark dashed line; n = 13).
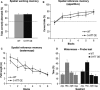
Figure 5
5‐
HT
transporter (5‐
HTT
) over‐expressing (5‐
HTTOE
) mice display normal spatial learning and memory. (A) Spatial working memory (non‐matching to place) rewarded alternation task. The graph shows the mean percent correct alternations (+
SEM
) from 40 trials. The dotted line indicates chance performance at 50% correct choices. Wild‐type (light bar; n = 10), 5‐
HTTOE
mice (dark bar; n = 11). (B) Appetitively motivated spatial reference memory Y‐maze task. The graph shows mean percent correct choices (±
SEM
) per block of 10 trials. Wild‐type (light solid line; n = 8), 5‐
HTTOE
mice (dark dashed line; n = 11). Horizontal dotted line indicates chance performance at 50% correct choices per block. (C) Morris water‐maze. The graph shows the mean distance swum [pathlength (m) ±
SEM
] to reach the hidden platform over six blocks of trials (four trials per block). Wild‐type (light solid line; n = 10), 5‐
HTTOE
mice (dark dashed line; n = 11). (D) Water‐maze probe test. Twenty‐four hours after block 6 of training, animals were returned to the pool with the platform removed and the amount of time spent swimming in each quadrant was recorded. Quadrant abbreviations: AdjL, adjacent left;
TRA
, training; AdjR, adjacent right; Opp, opposite. Mean percent time in quadrant (+
SEM
) is displayed. The dotted line indicates chance performance at 25% time spent in a given quadrant. Wild‐type (light bars; n = 10), 5‐
HTTOE
mice (dark bars; n = 11).
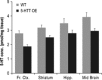
Figure 6
Reduced 5‐
HT
levels across a number of brain regions in 5‐
HT
transporter (5‐
HTT
) over‐expressing (5‐
HTTOE
) mice. Mean regional tissue 5‐
HT
concentration (+
SEM
) for wild‐types (light bars; n = 8) and 5‐
HTTOE
mice (dark bars; n = 10).
Similar articles
- SERT and uncertainty: serotonin transporter expression influences information processing biases for ambiguous aversive cues in mice.
McHugh SB, Barkus C, Lima J, Glover LR, Sharp T, Bannerman DM. McHugh SB, et al. Genes Brain Behav. 2015 Apr;14(4):330-6. doi: 10.1111/gbb.12215. Epub 2015 Apr 17. Genes Brain Behav. 2015. PMID: 25824641 Free PMC article. - Increased Serotonin Transporter Expression Reduces Fear and Recruitment of Parvalbumin Interneurons of the Amygdala.
Bocchio M, Fucsina G, Oikonomidis L, McHugh SB, Bannerman DM, Sharp T, Capogna M. Bocchio M, et al. Neuropsychopharmacology. 2015 Dec;40(13):3015-26. doi: 10.1038/npp.2015.157. Epub 2015 Jun 8. Neuropsychopharmacology. 2015. PMID: 26052039 Free PMC article. - Stress Enables Reinforcement-Elicited Serotonergic Consolidation of Fear Memory.
Baratta MV, Kodandaramaiah SB, Monahan PE, Yao J, Weber MD, Lin PA, Gisabella B, Petrossian N, Amat J, Kim K, Yang A, Forest CR, Boyden ES, Goosens KA. Baratta MV, et al. Biol Psychiatry. 2016 May 15;79(10):814-822. doi: 10.1016/j.biopsych.2015.06.025. Epub 2015 Jul 2. Biol Psychiatry. 2016. PMID: 26248536 Free PMC article. - Genetics of the serotonergic system in suicidal behavior.
Arango V, Huang YY, Underwood MD, Mann JJ. Arango V, et al. J Psychiatr Res. 2003 Sep-Oct;37(5):375-86. doi: 10.1016/s0022-3956(03)00048-7. J Psychiatr Res. 2003. PMID: 12849930 Review. - Learning, Reward, and Decision Making.
O'Doherty JP, Cockburn J, Pauli WM. O'Doherty JP, et al. Annu Rev Psychol. 2017 Jan 3;68:73-100. doi: 10.1146/annurev-psych-010416-044216. Epub 2016 Sep 28. Annu Rev Psychol. 2017. PMID: 27687119 Free PMC article. Review.
Cited by
- SERT and uncertainty: serotonin transporter expression influences information processing biases for ambiguous aversive cues in mice.
McHugh SB, Barkus C, Lima J, Glover LR, Sharp T, Bannerman DM. McHugh SB, et al. Genes Brain Behav. 2015 Apr;14(4):330-6. doi: 10.1111/gbb.12215. Epub 2015 Apr 17. Genes Brain Behav. 2015. PMID: 25824641 Free PMC article. - Increased Serotonin Transporter Expression Reduces Fear and Recruitment of Parvalbumin Interneurons of the Amygdala.
Bocchio M, Fucsina G, Oikonomidis L, McHugh SB, Bannerman DM, Sharp T, Capogna M. Bocchio M, et al. Neuropsychopharmacology. 2015 Dec;40(13):3015-26. doi: 10.1038/npp.2015.157. Epub 2015 Jun 8. Neuropsychopharmacology. 2015. PMID: 26052039 Free PMC article. - Neurobiology of Anorexia Nervosa: Serotonin Dysfunctions Link Self-Starvation with Body Image Disturbances through an Impaired Body Memory.
Riva G. Riva G. Front Hum Neurosci. 2016 Nov 24;10:600. doi: 10.3389/fnhum.2016.00600. eCollection 2016. Front Hum Neurosci. 2016. PMID: 27932968 Free PMC article. Review. - Serotonin, neural markers, and memory.
Meneses A. Meneses A. Front Pharmacol. 2015 Jul 21;6:143. doi: 10.3389/fphar.2015.00143. eCollection 2015. Front Pharmacol. 2015. PMID: 26257650 Free PMC article. Review. - Effect of selective serotonin reuptake inhibitor discontinuation on anxiety-like behaviours in mice.
Collins HM, Pinacho R, Ozdemir D, Bannerman DM, Sharp T. Collins HM, et al. J Psychopharmacol. 2022 Jul;36(7):794-805. doi: 10.1177/02698811221093032. Epub 2022 May 23. J Psychopharmacol. 2022. PMID: 35607713 Free PMC article.
References
- Adamec, R. , Burton, P. , Blundell, J. , Murphy, D.L. & Holmes, A. (2006) Vulnerability to mild predator stress in serotonin transporter knockout mice. Behav. Brain Res., 170, 126–140. - PubMed
- Barkus, C. , Line, S.J. , Huber, A. , Capitao, L. , Lima, J. , Jennings, K. , Lowry, J. , Sharp, T. , Bannerman, D.M. & McHugh, S.B. (2014) Variation in serotonin transporter expression modulates fear‐evoked hemodynamic responses and theta‐frequency neuronal oscillations in the amygdala. Biol. Psychiat., 75, 901–908. - PMC - PubMed
- Beck, A.T. (1976) Cognitive Therapy and the Emotional Disorders. Penguin Books, New York.
- Bizot, J. , Le Bihan, C. , Puech, A.J. , Hamon, M. & Thiebot, M. (1999) Serotonin and tolerance to delay of reward in rats. Psychopharmacology, 146, 400–412. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases