The impact of extracellular vesicle-encapsulated circulating microRNAs in lung cancer research - PubMed (original) (raw)
Review
The impact of extracellular vesicle-encapsulated circulating microRNAs in lung cancer research
Yu Fujita et al. Biomed Res Int. 2014.
Abstract
Lung cancer is the leading cause of cancer-related deaths. Biomarkers for lung cancer have raised great expectations in their clinical applications for early diagnosis, survival, and therapeutic responses. MicroRNAs (miRNAs), a family of short endogenous noncoding RNAs, play critical roles in cell growth, differentiation, and the development of various types of cancers. Current studies have shown that miRNAs are present in the extracellular spaces, packaged into various membrane-bound vesicles. Tumor-specific circulating miRNAs have been developed as early diagnostic biomarkers for lung cancer. Remarkably, some studies have succeeded in discovering circulating miRNAs with prognostic or predictive significance. Extracellular vesicles (EVs), such as exosomes and microvesicles, are recognized as novel tools for cell-cell communication and as biomarkers for various diseases. Their vesicle composition and miRNA content have the ability to transfer biological information to recipient cells and play an important role in cancer metastasis and prognosis. This review provides an in-depth summary of current findings on circulating miRNAs in lung cancer patients used as diagnostic biomarkers. We also discuss the role of EV miRNAs in cell-cell communication and explore the effectiveness of these contents as predictive biomarkers for cancer malignancy.
Figures
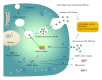
Figure 1
MiRNA release mechanisms into extracellular space. Precursor miRNAs are processed by ribonuclease Dicer to mature double-stranded miRNAs (miRNA duplex). One strand of the miRNA duplex is selectively loaded into the RNA-induced silencing complex (RISC), which contains the Argonaute (AGO) family protein as a core component. A fraction of miRNAs are released from living cells into the extracellular environment via the following mechanisms: (1) sorting into multivesicular bodies (MVB) and secretion via exosomes, (2) incorporating into microvesicles that are formed by the outward shedding of the plasma membrane, (3) associating with RNA-binding proteins, such as AGO2 and release of the miRNA-AGO complexes, and (4) exporting and incorporating into high-density lipoprotein (HDL) particles. Extracellular vesicle miRNAs are possibly involved in cell-cell communication.
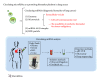
Figure 2
Circulating microRNAs as a promising biomarker platform in lung cancer. Circulating miRNAs have been found to be packaged into various membrane-bound vesicles, such as exosomes and microvesicles and to exist in a vesicle-free form associated with protein or high-density lipoprotein complexes. Extracellular vesicles (EVs), such as exosomes and microvesicles, are recognized as novel tools for cell-cell communication and might be predictive biomarkers for cancer malignancy. EVs that circulate in body fluids can be readily recovered using several existing isolation methods and analyzed by various detection methods.
Similar articles
- Circulating microRNAs as diagnostic and prognostic tools for hepatocellular carcinoma.
Zhang YC, Xu Z, Zhang TF, Wang YL. Zhang YC, et al. World J Gastroenterol. 2015 Sep 14;21(34):9853-62. doi: 10.3748/wjg.v21.i34.9853. World J Gastroenterol. 2015. PMID: 26379392 Free PMC article. Review. - Circulating or tissue microRNAs and extracellular vesicles as potential lung cancer biomarkers: a systematic review.
Song Y, Yu X, Zang Z, Zhao G. Song Y, et al. Int J Biol Markers. 2018 Jan;33(1):3-9. doi: 10.5301/ijbm.5000307. Int J Biol Markers. 2018. PMID: 29076520 - Intercellular communication by extracellular vesicles and their microRNAs in asthma.
Fujita Y, Yoshioka Y, Ito S, Araya J, Kuwano K, Ochiya T. Fujita Y, et al. Clin Ther. 2014 Jun 1;36(6):873-81. doi: 10.1016/j.clinthera.2014.05.006. Epub 2014 Jun 6. Clin Ther. 2014. PMID: 24909737 - Clinical significance of circulating miRNA detection in lung cancer.
Zhao C, Lu F, Chen H, Zhao F, Zhu Z, Zhao X, Chen H. Zhao C, et al. Med Oncol. 2016 May;33(5):41. doi: 10.1007/s12032-016-0757-5. Epub 2016 Mar 31. Med Oncol. 2016. PMID: 27034265 Review. - Clinical relevance of circulating cell-free microRNAs in ovarian cancer.
Nakamura K, Sawada K, Yoshimura A, Kinose Y, Nakatsuka E, Kimura T. Nakamura K, et al. Mol Cancer. 2016 Jun 24;15(1):48. doi: 10.1186/s12943-016-0536-0. Mol Cancer. 2016. PMID: 27343009 Free PMC article. Review.
Cited by
- Advancements and trends in exosome research in lung cancer from a bibliometric analysis (2004-2023).
Zhong W, Zhao X, Zhang X, Xu Y, Liu M, Yang X, Jiang Y, Shen X. Zhong W, et al. Front Oncol. 2024 Apr 16;14:1358101. doi: 10.3389/fonc.2024.1358101. eCollection 2024. Front Oncol. 2024. PMID: 38690166 Free PMC article. - Extracellular Vesicle: An Unknown Environmental Factor for Causing Airway Disease.
Pyun BY. Pyun BY. Allergy Asthma Immunol Res. 2016 May;8(3):179-80. doi: 10.4168/aair.2016.8.3.179. Allergy Asthma Immunol Res. 2016. PMID: 26922926 Free PMC article. No abstract available. - Analysis of MicroRNA Cargo in Circulating Extracellular Vesicles from HIV-Infected Individuals with Pulmonary Hypertension.
Mahajan A, Gunewardena S, Morris A, Clauss M, Dhillon NK. Mahajan A, et al. Cells. 2024 May 21;13(11):886. doi: 10.3390/cells13110886. Cells. 2024. PMID: 38891019 Free PMC article. - Quantitative Analysis of Exosomes From Murine Lung Cancer Cells by Flow Cytometry.
Rim KT, Kim SJ. Rim KT, et al. J Cancer Prev. 2016 Sep;21(3):194-200. doi: 10.15430/JCP.2016.21.3.194. Epub 2016 Sep 30. J Cancer Prev. 2016. PMID: 27722146 Free PMC article. - Application of atomic force microscopy in cancer research.
Deng X, Xiong F, Li X, Xiang B, Li Z, Wu X, Guo C, Li X, Li Y, Li G, Xiong W, Zeng Z. Deng X, et al. J Nanobiotechnology. 2018 Dec 11;16(1):102. doi: 10.1186/s12951-018-0428-0. J Nanobiotechnology. 2018. PMID: 30538002 Free PMC article. Review.
References
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA: Cancer Journal for Clinicians. 2013;63(1):11–30. - PubMed
- Cullen BR. Transcription and processing of human microRNA precursors. Molecular Cell. 2004;16(6):861–865. - PubMed
- Lee Y, Ahn C, Han J, et al. The nuclear RNase III Drosha initiates microRNA processing. Nature. 2003;425(6956):415–419. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical