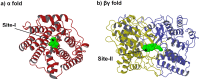Predicting the functions and specificity of triterpenoid synthases: a mechanism-based multi-intermediate docking approach - PubMed (original) (raw)
Predicting the functions and specificity of triterpenoid synthases: a mechanism-based multi-intermediate docking approach
Bo-Xue Tian et al. PLoS Comput Biol. 2014.
Abstract
Terpenoid synthases construct the carbon skeletons of tens of thousands of natural products. To predict functions and specificity of triterpenoid synthases, a mechanism-based, multi-intermediate docking approach is proposed. In addition to enzyme function prediction, other potential applications of the current approach, such as enzyme mechanistic studies and enzyme redesign by mutagenesis, are discussed.
Conflict of interest statement
MPJ is a consultant for Schrödinger LLC, which licenses, develops, and distributes software used in this work. All other authors have declared that no competing interests exist.
Figures
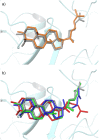
Figure 1. Example structures of TPSs: a) limonene synthase (PDB: 2ONH) ; b) squalene-hopene cyclase (PDB: 1SQC) , .
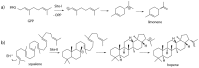
Figure 2. Example reactions of TPSs: a) limonene synthase; b) squalene-hopene cyclase.
Figure 3. Reaction channels for triterpenoid synthase and triterpenoid synthase-like enzymes , .
Figure 4. Sequence similarity network of triterpenoid synthase and triterpenoid synthase-like proteins colored by reaction channels.
Each node represents a protein sequence, and nodes are connected when the Blast _E_-value for the pair of sequences is more significant than 10−60 (panel a) or 10−220/10−300 (panel b). Gray nodes represent enzymes lacking annotations in the manually curated portion of UniProtKB (Swiss-Prot), i.e., likely to be experimentally uncharacterized.
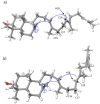
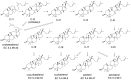
Figure 5. Illustration of the key dihedral angle C16-C17-C18-H18 that determines the conversion of I1 to I2: a) A-I1; b) B-I1.
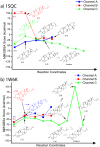
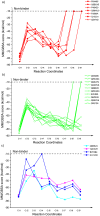
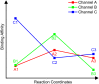
Figure 6. Carbocationic intermediate docking scores (MM/GBSA) along the reaction coordinates of a) 1SQC and b) 1W6K.
We arbitrarily assigned a score of +100 kcal/mol to intermediates that could not be successfully docked.
Figure 7. a) Superimposed view of the product lanosterol in the 1W6K crystal structure (grey) and the docking pose of C-I6 (the product precursor carbocation, c.f. Figure 6b ; in orange); b) The docking poses of the second representative intermediates: A-I2 (blue), B-I2 (red) and C-I2 (lime), as well as lanosterol in the 1W6K crystal structure (grey, c.f. Figure 6b ).
Figure 8. Intermediates and products of Channel C.
Figure 9. Docking score (MM/GBSA) of 9 carbocationic intermediates for 22 triterpenoid synthase homology models that follow channel C.
Compounds that could not be successfully docked at all are arbitrarily assigned a docking score of −10 kcal/mol. Figure legend shows the UniProtKB IDs for the triterpenoid synthases. Panel a shows the docking scores against 8 lanosterol synthases (in red); panel b shows the docking scores against 10 cycloartenol synthases (in lime green); and panel c shows the docking scores against a cucurbitadienol synthase (in cyan), a parkeol synthase (in magenta) and 2 protostadienol synthases (in blue). Details c.f. Table S2.
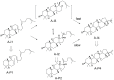
Figure 10. Key intermediates involved in the reaction channel leading to the hopanyl cation (A-I4), and products derived from these.
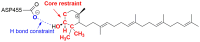
Figure 11. Example of constraints and restraints used during docking (residue numbering is for 1W6K).
Figure 12. A hypothetical example output of the carbocation docking.
Similar articles
- Defining the Product Chemical Space of Monoterpenoid Synthases.
Tian B, Poulter CD, Jacobson MP. Tian B, et al. PLoS Comput Biol. 2016 Aug 12;12(8):e1005053. doi: 10.1371/journal.pcbi.1005053. eCollection 2016 Aug. PLoS Comput Biol. 2016. PMID: 27517297 Free PMC article. - Structural and Chemical Biology of Terpenoid Cyclases.
Christianson DW. Christianson DW. Chem Rev. 2017 Sep 13;117(17):11570-11648. doi: 10.1021/acs.chemrev.7b00287. Epub 2017 Aug 25. Chem Rev. 2017. PMID: 28841019 Free PMC article. Review. - Unearthing the roots of the terpenome.
Christianson DW. Christianson DW. Curr Opin Chem Biol. 2008 Apr;12(2):141-50. doi: 10.1016/j.cbpa.2007.12.008. Epub 2008 Feb 20. Curr Opin Chem Biol. 2008. PMID: 18249199 Free PMC article. Review. - Mutagenesis approaches to deduce structure-function relationships in terpene synthases.
Segura MJ, Jackson BE, Matsuda SP. Segura MJ, et al. Nat Prod Rep. 2003 Jun;20(3):304-17. doi: 10.1039/b008338k. Nat Prod Rep. 2003. PMID: 12828369 Review. - Analyzing and Engineering the Product Selectivity of a 2-Methylenebornane Synthase.
Kschowak MJ, Maier F, Wortmann H, Buchhaupt M. Kschowak MJ, et al. ACS Synth Biol. 2020 May 15;9(5):981-986. doi: 10.1021/acssynbio.9b00432. Epub 2020 May 4. ACS Synth Biol. 2020. PMID: 32364702
Cited by
- Terpene Synthases as Metabolic Gatekeepers in the Evolution of Plant Terpenoid Chemical Diversity.
Karunanithi PS, Zerbe P. Karunanithi PS, et al. Front Plant Sci. 2019 Oct 1;10:1166. doi: 10.3389/fpls.2019.01166. eCollection 2019. Front Plant Sci. 2019. PMID: 31632418 Free PMC article. Review. - Crystal Structure of Cucumene Synthase, a Terpenoid Cyclase That Generates a Linear Triquinane Sesquiterpene.
Blank PN, Pemberton TA, Chow JY, Poulter CD, Christianson DW. Blank PN, et al. Biochemistry. 2018 Nov 6;57(44):6326-6335. doi: 10.1021/acs.biochem.8b00899. Epub 2018 Oct 22. Biochemistry. 2018. PMID: 30346736 Free PMC article. - Computational-guided discovery and characterization of a sesquiterpene synthase from Streptomyces clavuligerus.
Chow JY, Tian BX, Ramamoorthy G, Hillerich BS, Seidel RD, Almo SC, Jacobson MP, Poulter CD. Chow JY, et al. Proc Natl Acad Sci U S A. 2015 May 5;112(18):5661-6. doi: 10.1073/pnas.1505127112. Epub 2015 Apr 21. Proc Natl Acad Sci U S A. 2015. PMID: 25901324 Free PMC article. - Modulation of inherent dynamical tendencies of the bisabolyl cation via preorganization in _epi_-isozizaene synthase.
Pemberton RP, Ho KC, Tantillo DJ. Pemberton RP, et al. Chem Sci. 2015 Apr 1;6(4):2347-2353. doi: 10.1039/c4sc03782k. Epub 2015 Feb 2. Chem Sci. 2015. PMID: 29308148 Free PMC article. - Defining the Product Chemical Space of Monoterpenoid Synthases.
Tian B, Poulter CD, Jacobson MP. Tian B, et al. PLoS Comput Biol. 2016 Aug 12;12(8):e1005053. doi: 10.1371/journal.pcbi.1005053. eCollection 2016 Aug. PLoS Comput Biol. 2016. PMID: 27517297 Free PMC article.
References
- Birch AJ (1957) The Chemistry of Terpenoid Compounds. Nature 180: 470–471.
- Sacchettini JC, Poulter CD (1997) Biochemistry - Creating isoprenoid diversity. Science 277: 1788–1789. - PubMed
- Christianson DW (2006) Structural biology and chemistry of the terpenoid cyclases. Chem Rev 106: 3412–3142. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources