Easy quantitative assessment of genome editing by sequence trace decomposition - PubMed (original) (raw)
Easy quantitative assessment of genome editing by sequence trace decomposition
Eva K Brinkman et al. Nucleic Acids Res. 2014.
Abstract
The efficacy and the mutation spectrum of genome editing methods can vary substantially depending on the targeted sequence. A simple, quick assay to accurately characterize and quantify the induced mutations is therefore needed. Here we present TIDE, a method for this purpose that requires only a pair of PCR reactions and two standard capillary sequencing runs. The sequence traces are then analyzed by a specially developed decomposition algorithm that identifies the major induced mutations in the projected editing site and accurately determines their frequency in a cell population. This method is cost-effective and quick, and it provides much more detailed information than current enzyme-based assays. An interactive web tool for automated decomposition of the sequence traces is available. TIDE greatly facilitates the testing and rational design of genome editing strategies.
© The Author(s) 2014. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures
Figure 1.
Assessment of genome editing by sequence trace decomposition. (a) Due to imperfect repair after cutting by a targeted nuclease, the DNA in the cell pool consists of a mixture of indels, which yields a composite sequence trace after the break site. (b) Overview of TIDE algorithm and output, which consists of three main steps: (1) Visualization of aberrant sequence signal in control (black) and treated sample (green), the expected break site (vertical dotted line) and the region used for decomposition (gray bar); (2) Decomposition yielding the spectrum of indels and their frequencies; (3) Inference of the base composition of +1 insertions. See main text and
for explanation.
Figure 2.
Proof-of-principle of TIDE. (a) A DNA fragment carrying a +1 insertion was mixed in indicated relative amounts with a corresponding wild-type DNA fragment (horizontal axis), after which the +1 insertion content was determined by TIDE (vertical axis) using the default search for indels with a size range of 0.10. Inset: relative abundance of the inserted nucleotide in a wt, +1 mix (90%:10%). See Supplementary Figure S1 for the complete decomposition results. (b) TIDE decomposition of various complex mixtures of wild-type DNA with DNA carrying a range of indels. See also Supplementary Figure S2a–c.
Figure 3.
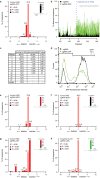
Application of TIDE to in vivo edited DNA sequences. (a–d) A pool of human K562 cells expressing GFP treated with Cas9 alone (control) and cells treated with Cas9 and a GFP targeting sgRNA (sample) were analyzed by: TIDE (a and b), sequence analysis of 84 cloned DNA fragments (c) and flow cytometry (d). (a) Indel spectrum determined by TIDE. Inset shows the estimated composition of the inserted base for the +1 insertion. (b) Aberrant nucleotide signal of the sample (green) compared to that of the control (black). Blue dotted line indicates the expected cutting site. Gray horizontal bar shows the region used for decomposition. (c) Comparison of indel occurrences in cloned DNA fragments (n = 84) to frequencies estimated by TIDE, with _P_-values according to Pearson's chi-squared test. Decomposition was limited to indels of size 0-10, hence larger indels could not be detected. (d) Distributions of GFP fluorescence intensities of Cas9 and Cas9+sgRNA treated cells, measured by flow cytometry. The percentage of GFP-positive cells is indicated in the top right corner within indicated histogram gate. (e–h) TIDE analysis of various endogenous genes (NDC1, LBR, LMN) targeted with RGENs in human cell lines (K562, RPE) and in a Drosophila cell line (Kc167). Insets: prediction of the inserted base for +1 insertions.
Similar articles
- Rapid Quantitative Evaluation of CRISPR Genome Editing by TIDE and TIDER.
Brinkman EK, van Steensel B. Brinkman EK, et al. Methods Mol Biol. 2019;1961:29-44. doi: 10.1007/978-1-4939-9170-9_3. Methods Mol Biol. 2019. PMID: 30912038 - Poly peak parser: Method and software for identification of unknown indels using sanger sequencing of polymerase chain reaction products.
Hill JT, Demarest BL, Bisgrove BW, Su YC, Smith M, Yost HJ. Hill JT, et al. Dev Dyn. 2014 Dec;243(12):1632-6. doi: 10.1002/dvdy.24183. Epub 2014 Sep 30. Dev Dyn. 2014. PMID: 25160973 Free PMC article. - Fast and sensitive detection of indels induced by precise gene targeting.
Yang Z, Steentoft C, Hauge C, Hansen L, Thomsen AL, Niola F, Vester-Christensen MB, Frödin M, Clausen H, Wandall HH, Bennett EP. Yang Z, et al. Nucleic Acids Res. 2015 May 19;43(9):e59. doi: 10.1093/nar/gkv126. Epub 2015 Mar 9. Nucleic Acids Res. 2015. PMID: 25753669 Free PMC article. - CRISPR/Cas genome editing to optimize pharmacologically active plant natural products.
Dey A. Dey A. Pharmacol Res. 2021 Feb;164:105359. doi: 10.1016/j.phrs.2020.105359. Epub 2020 Dec 4. Pharmacol Res. 2021. PMID: 33285226 Review. - Harnessing CRISPR-Cas systems for bacterial genome editing.
Selle K, Barrangou R. Selle K, et al. Trends Microbiol. 2015 Apr;23(4):225-32. doi: 10.1016/j.tim.2015.01.008. Epub 2015 Feb 17. Trends Microbiol. 2015. PMID: 25698413 Review.
Cited by
- Agrobacterium-Mediated Transformation and Targeted Mutagenesis Using SpCas12f in Rice.
Sukegawa S, Toki S, Saika H. Sukegawa S, et al. Methods Mol Biol. 2025;2869:75-90. doi: 10.1007/978-1-0716-4204-7_9. Methods Mol Biol. 2025. PMID: 39499469 - Regulation of Sex-biased Gene Expression by the Ancestral X-Y Chromosomal Gene Pair Kdm5c-Kdm5d.
Malcore RM, Samanta MK, Kalantry S, Iwase S. Malcore RM, et al. bioRxiv [Preprint]. 2024 Oct 27:2024.10.24.620066. doi: 10.1101/2024.10.24.620066. bioRxiv. 2024. PMID: 39484414 Free PMC article. Preprint. - A resurrected ancestor of Cas12a expands target access and substrate recognition for nucleic acid editing and detection.
Jabalera Y, Tascón I, Samperio S, López-Alonso JP, Gonzalez-Lopez M, Aransay AM, Abascal-Palacios G, Beisel CL, Ubarretxena-Belandia I, Perez-Jimenez R. Jabalera Y, et al. Nat Biotechnol. 2024 Oct 31. doi: 10.1038/s41587-024-02461-3. Online ahead of print. Nat Biotechnol. 2024. PMID: 39482449 - Decisive role of mDia-family formins in cell cortex function of highly adherent cells.
Scholz J, Stephan T, Pérez AG, Csiszár A, Hersch N, Fischer LS, Brühmann S, Körber S, Litschko C, Mijanovic L, Kaufmann T, Lange F, Springer R, Pich A, Jakobs S, Peckham M, Tarantola M, Grashoff C, Merkel R, Faix J. Scholz J, et al. Sci Adv. 2024 Nov;10(44):eadp5929. doi: 10.1126/sciadv.adp5929. Epub 2024 Oct 30. Sci Adv. 2024. PMID: 39475610 Free PMC article.
References
- Kim H., Kim J.S. A guide to genome engineering with programmable nucleases. Nat. Rev. Genet. 2014;15:321–334. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials