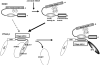Novel mechanisms of endothelial mechanotransduction - PubMed (original) (raw)
Review
Novel mechanisms of endothelial mechanotransduction
Jun-ichi Abe et al. Arterioscler Thromb Vasc Biol. 2014 Nov.
Abstract
Atherosclerosis is a focal disease that develops preferentially where nonlaminar, disturbed blood flow occurs, such as branches, bifurcations, and curvatures of large arteries. Endothelial cells sense and respond differently to disturbed flow compared with steady laminar flow. Disturbed flow that occurs in so-called atheroprone areas activates proinflammatory and apoptotic signaling, and this results in endothelial dysfunction and leads to subsequent development of atherosclerosis. In contrast, steady laminar flow as atheroprotective flow promotes expression of many anti-inflammatory genes, such as Kruppel-like factor 2 and endothelial nitric oxide synthase and inhibits endothelial inflammation and athrogenesis. Here we will discuss that disturbed flow and steady laminar flow induce pro- and antiatherogenic events via flow type-specific mechanotransduction pathways. We will focus on 5 mechanosensitive pathways: mitogen-activated protein kinases/extracellular signal-regulated kinase 5/Kruppel-like factor 2 signaling, extracellular signal-regulated kinase/peroxisome proliferator-activated receptor signaling, and mechanosignaling pathways involving SUMOylation, protein kinase C-ζ, and p90 ribosomal S6 kinase. We think that clarifying regulation mechanisms between these 2 flow types will provide new insights into therapeutic approaches for the prevention and treatment of atherosclerosis.
Keywords: ERK5; PKCζ; SUMOylation; flow.
© 2014 American Heart Association, Inc.
Figures
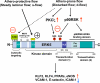
Figure 1. Primary structure of ERK5 and its regulation
The N-terminus region with SUMO modification inhibits its own transactivation. After ERK5 kinase activation induced by MEK5 binding and TEY motif phosphorylation with de-SUMOylation of K6/K22 sites, ERK5 transcriptional activity at the C-terminus region is fully activated. In contrast athero-prone flow increases ERK5-SUMOylation and ERK5 S496 phosphorylation and inhibits ERK5 transcriptional activity.
Figure 2. Model for the ERK5-PPARγ interaction-mediated PPARγ transactivation
The position of Helix 12 is regulated by ligand binding. When the PPARγ ligand binds to the receptor, Helix 12 folds back to form a part of the co-activator binding surface, and inhibits corepressor (such as SMRT) binding to PPARγ. The co-repressor interaction surface requires Helix 3-5. We found a critical role of the PPARγ hinge-helix 1 domain in ERK5-mediated PPARγ transactivation. The inactive N-terminus kinase domain of ERK5 inhibits its own transactivation and PPARγ binding. After ERK5 activation the inhibitory effect of the N-terminus domain decreases, and subsequently the middle region can fully interact with the hinge-helix 1 region of PPARγ. The association of ERK5 with the hinge-helix 1 region of PPARγ releases co-repressor of SMRT and induces full activation of PPARγ. AF-1/2: Activating function (AF)-1/2 transactivation domain, DBD: DNA binding domain. Reprinted and modified from Akaike et al.
Figure 3. The regulation of SUMOylation pathway
Protein SUMOylation is achieved by a recycle system consisting of conjugation and deconjugation pathway. SUMO conjugation to a target substrate requires an enzymatic cascade, which involves three classes of enzymes (E1→E2→E3). The sentrin/SUMO-specific proteases (SENPs) are responsible for the deconjugation pathway as well as the maturation process of newly synthesized SUMO protein. The primary subcellular localization of each SENP is also listed. Reprinted and modified from Woo et al. with permission of the publisher.
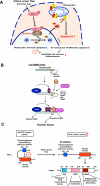
Figure 4. Athero-prone flow increases p53 SUMOylation via PKCζ-PIAS4 binding
(A) Athero-prone flow uniquely activates PKCζ, which increases PKCζ-PIAS4 binding and PIAS4 SUMO E3 ligase activity, and subsequently increases p53 SUMOylation. SUMOylation causes p53 nuclear export and binds to Bcl-2, which inhibits anti-apoptotic function of Bcl-2 and increases apoptosis. (B) p53 nuclear export and stabilization. Masking of the C-terminus NES results in nuclear localization of unmodified p53, but a low level of ubiquitination by MDM2 exposes the NES, causing the p53-Ub fusion protein to come out of the nucleus. When MDM2 levels are low, ubiquitination promotes the interaction of p53 with PIAS4 and further modification of p53 by SUMOylation that causes the release of MDM2 and nuclear export, which may increase the cytoplasmic apoptotic function of p53. Under conditions of high MDM2, persistent binding and activity of MDM2 leads to polyubiquitination and degradation of p53. These data suggest important roles of SUMOylation in p53 stabilization, localization, and subsequent apoptosis. Reprinted and modified from Carter et al with permission of the publisher. (C) PKCζ-mediated p53 SUMOylation requires PKCζ-PIAS4 binding at SP-RING domain, not PKCζ-mediated phosphorylation of PIAS4. SAP: scaffold attachment factor-A/B, acinus and PIAS domain; SP-RING: Siz/PIAS-RING domain.
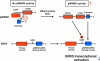
Figure 5. Athero-prone flow increases p90RSK activation, leading to p90RSK-ERK5 association and ERK5 S496 phosphorylation, and subsequently decreases in ERK5 transcriptional activity
At the basal level, inactive p90RSK inhibits the D-domain to bind ERK5. Once p90RSK is activated, the inhibition of the kinase domain is released and the D-domain of p90RSK associates with the ERK5 C-terminus domain and increases ERK5 S496 phosphorylation, which inhibits ERK5 transcriptional activity. NTKD: NH2-terminal kinase domain, CTKD: COOH-terminal kinase domain, D-domain: NH2-terminal docking domain.
Similar articles
- Disturbed-flow-mediated vascular reactive oxygen species induce endothelial dysfunction.
Heo KS, Fujiwara K, Abe J. Heo KS, et al. Circ J. 2011;75(12):2722-30. doi: 10.1253/circj.cj-11-1124. Epub 2011 Nov 10. Circ J. 2011. PMID: 22076424 Free PMC article. Review. - Laminar flow activation of ERK5 protein in vascular endothelium leads to atheroprotective effect via NF-E2-related factor 2 (Nrf2) activation.
Kim M, Kim S, Lim JH, Lee C, Choi HC, Woo CH. Kim M, et al. J Biol Chem. 2012 Nov 23;287(48):40722-31. doi: 10.1074/jbc.M112.381509. Epub 2012 Oct 5. J Biol Chem. 2012. PMID: 23043106 Free PMC article. - De-SUMOylation enzyme of sentrin/SUMO-specific protease 2 regulates disturbed flow-induced SUMOylation of ERK5 and p53 that leads to endothelial dysfunction and atherosclerosis.
Heo KS, Chang E, Le NT, Cushman H, Yeh ET, Fujiwara K, Abe J. Heo KS, et al. Circ Res. 2013 Mar 15;112(6):911-23. doi: 10.1161/CIRCRESAHA.111.300179. Epub 2013 Feb 4. Circ Res. 2013. PMID: 23381569 Free PMC article. - Shear stress and atherosclerosis.
Heo KS, Fujiwara K, Abe J. Heo KS, et al. Mol Cells. 2014 Jun;37(6):435-40. doi: 10.14348/molcells.2014.0078. Epub 2014 Apr 30. Mol Cells. 2014. PMID: 24781409 Free PMC article. Review. - Extracellular signal-regulated kinase 5 SUMOylation antagonizes shear stress-induced antiinflammatory response and endothelial nitric oxide synthase expression in endothelial cells.
Woo CH, Shishido T, McClain C, Lim JH, Li JD, Yang J, Yan C, Abe J. Woo CH, et al. Circ Res. 2008 Mar 14;102(5):538-45. doi: 10.1161/CIRCRESAHA.107.156877. Epub 2008 Jan 24. Circ Res. 2008. PMID: 18218985
Cited by
- Small Rho GTPase-mediated actin dynamics at endothelial adherens junctions.
van Buul JD, Timmerman I. van Buul JD, et al. Small GTPases. 2016;7(1):21-31. doi: 10.1080/21541248.2015.1131802. Epub 2016 Jan 29. Small GTPases. 2016. PMID: 26825121 Free PMC article. - Hypoxia Triggers SENP1 (Sentrin-Specific Protease 1) Modulation of KLF15 (Kruppel-Like Factor 15) and Transcriptional Regulation of Arg2 (Arginase 2) in Pulmonary Endothelium.
Pandey D, Nomura Y, Rossberg MC, Hori D, Bhatta A, Keceli G, Leucker T, Santhanam L, Shimoda LA, Berkowitz D, Romer L. Pandey D, et al. Arterioscler Thromb Vasc Biol. 2018 Apr;38(4):913-926. doi: 10.1161/ATVBAHA.117.310660. Epub 2018 Feb 22. Arterioscler Thromb Vasc Biol. 2018. PMID: 29472234 Free PMC article. - Regulation of Kir2.1 Function Under Shear Stress and Cholesterol Loading.
Le NT, Abe JI. Le NT, et al. J Am Heart Assoc. 2018 Mar 3;7(5):e008749. doi: 10.1161/JAHA.118.008749. J Am Heart Assoc. 2018. PMID: 29502108 Free PMC article. No abstract available. - Endothelial Cell Dysfunction and the Pathobiology of Atherosclerosis.
Gimbrone MA Jr, García-Cardeña G. Gimbrone MA Jr, et al. Circ Res. 2016 Feb 19;118(4):620-36. doi: 10.1161/CIRCRESAHA.115.306301. Circ Res. 2016. PMID: 26892962 Free PMC article. Review. - The roles of Hippo/YAP signaling pathway in physical therapy.
Pan C, Hao X, Deng X, Lu F, Liu J, Hou W, Xu T. Pan C, et al. Cell Death Discov. 2024 Apr 26;10(1):197. doi: 10.1038/s41420-024-01972-x. Cell Death Discov. 2024. PMID: 38670949 Free PMC article. Review.
References
- Won D, Zhu SN, Chen M, Teichert AM, Fish JE, Matouk CC, Bonert M, Ojha M, Marsden PA, Cybulsky MI. Relative reduction of endothelial nitric-oxide synthase expression and transcription in atherosclerosis-prone regions of the mouse aorta and in an in vitro model of disturbed flow. The American journal of pathology. 2007;171:1691–1704. - PMC - PubMed
- Bao X, Lu C, Frangos JA. Temporal gradient in shear but not steady shear stress induces pdgf-a and mcp-1 expression in endothelial cells: Role of no, nf kappa b, and egr-1. Arterioscler Thromb Vasc Biol. 1999;19:996–1003. - PubMed
- Wang GX, Cai SX, Wang PQ, Ouyang KQ, Wang YL, Xu SR. Shear-induced changes in endothelin-1 secretion of microvascular endothelial cells. Microvasc Res. 2002;63:209–217. - PubMed
- Hsiai TK, Cho SK, Reddy S, Hama S, Navab M, Demer LL, Honda HM, Ho CM. Pulsatile flow regulates monocyte adhesion to oxidized lipid-induced endothelial cells. Arteriosclerosis, thrombosis, and vascular biology. 2001;21:1770–1776. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HL 106158/HL/NHLBI NIH HHS/United States
- HL-108551/HL/NHLBI NIH HHS/United States
- R01 HL064839/HL/NHLBI NIH HHS/United States
- R01 HL102746/HL/NHLBI NIH HHS/United States
- HL-102746/HL/NHLBI NIH HHS/United States
- HL-064839/HL/NHLBI NIH HHS/United States
- R01 HL106158/HL/NHLBI NIH HHS/United States
- R01 HL108551/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous